| |||
| Names | |||
|---|---|---|---|
| Other names Ammonium hypophosphite | |||
| Identifiers | |||
3D model (JSmol) | |||
| ChemSpider | |||
| ECHA InfoCard | 100.029.333 | ||
| EC Number |
| ||
CompTox Dashboard (EPA) | |||
| |||
| |||
| Properties | |||
| H6NO2P | |||
| Molar mass | 83.027 g·mol−1 | ||
| Appearance | colorless crystals | ||
| Density | 1.634 g/cm3 | ||
| Melting point | 200 | ||
| soluble | |||
| Hazards | |||
| GHS labelling: | |||
 [1] [1] | |||
| Warning | |||
| H315, H319, H335 [2] | |||
| P261, P305, P338, P351 [2] | |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
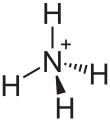
Ammonium phosphinate is a chemical compound with the chemical formula NH4PH2O2. [3] [4] This is a salt of ammonium and phosphinic acid.