
Cadmium sulfide is the inorganic compound with the formula CdS. Cadmium sulfide is a yellow salt. It occurs in nature with two different crystal structures as the rare minerals greenockite and hawleyite, but is more prevalent as an impurity substituent in the similarly structured zinc ores sphalerite and wurtzite, which are the major economic sources of cadmium. As a compound that is easy to isolate and purify, it is the principal source of cadmium for all commercial applications. Its vivid yellow color led to its adoption as a pigment for the yellow paint "cadmium yellow" in the 1800s.

Mercury sulfide, or mercury(II) sulfide is a chemical compound composed of the chemical elements mercury and sulfur. It is represented by the chemical formula HgS. It is virtually insoluble in water.
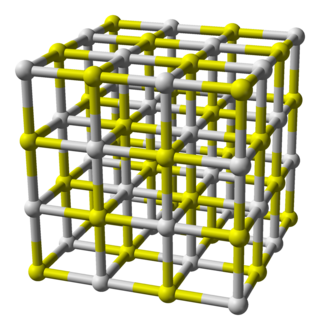
Calcium sulfide is the chemical compound with the formula CaS. This white material crystallizes in cubes like rock salt. CaS has been studied as a component in a process that would recycle gypsum, a product of flue-gas desulfurization. Like many salts containing sulfide ions, CaS typically has an odour of H2S, which results from small amount of this gas formed by hydrolysis of the salt.

Ammonium hydrosulfide is the chemical compound with the formula [NH4]SH.

Tetrasulfur tetranitride is an inorganic compound with the formula S4N4. This vivid orange, opaque, crystalline explosive is the most important binary sulfur nitride, which are compounds that contain only the elements sulfur and nitrogen. It is a precursor to many S-N compounds and has attracted wide interest for its unusual structure and bonding.
An inorganic nonaqueous solvent is a solvent other than water, that is not an organic compound. These solvents are used in chemical research and industry for reactions that cannot occur in aqueous solutions or require a special environment. Inorganic nonaqueous solvents can be classified into two groups, protic solvents and aprotic solvents. Early studies on inorganic nonaqueous solvents evaluated ammonia, hydrogen fluoride, sulfuric acid, as well as more specialized solvents, hydrazine, and selenium oxychloride.
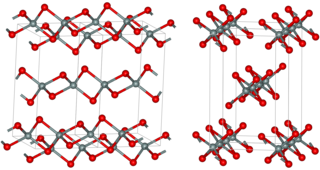
Silicon disulfide is the inorganic compound with the formula SiS2. Like silicon dioxide, this material is polymeric, but it adopts a 1-dimensional structure quite different from the usual forms of SiO2.

Ammonium dichromate is an inorganic compound with the formula (NH4)2Cr2O7. In this compound, as in all chromates and dichromates, chromium is in a +6 oxidation state, commonly known as hexavalent chromium. It is a salt consisting of ammonium ions and dichromate ions.

Ammonium tetrathiomolybdate is the chemical compound with the formula (NH4)2MoS4. This bright red ammonium salt is an important reagent in the chemistry of molybdenum and has been used as a building block in bioinorganic chemistry. The thiometallate (see metallate) anion has the distinctive property of undergoing oxidation at the sulfur centers concomitant with reduction of the metal from Mo(VI) to Mo(IV).
Thiosulfuric acid is the inorganic compound with the formula H2S2O3. It has attracted academic interest as a simple, easily accessed compound that is labile. It has few practical uses.

Ammonium thiocyanate is an inorganic compound with the formula [NH4]+[SCN]−. It is an ammonium salt of thiocyanic acid. It consists of ammonium cations [NH4]+ and thiocyanate anions [SCN]−.

Thiophosphoryl fluoride is an inorganic molecular gas with formula PSF3 containing phosphorus, sulfur and fluorine. It spontaneously ignites in air and burns with a cool flame. The discoverers were able to have flames around their hands without discomfort, and called it "probably one of the coldest flames known". The gas was discovered in 1888.

Gallium(III) sulfide, Ga2S3, is a compound of sulfur and gallium, that is a semiconductor that has applications in electronics and photonics.
Tungsten trisulfide is an inorganic compound of tungsten and sulfur with the chemical formula WS3. The compound looks like chocolate-brown powder.

Chromium(II) sulfide is an inorganic compound of chromium and sulfur with the chemical formula CrS. The compound forms black hexagonal crystals, insoluble in water.
Polonium sulfide is an inorganic compound of polonium and sulfur with the chemical formula PoS. The compound is radioactive and forms black crystals.
Neodymium(III) sulfide is a inorganic chemical compound with the formula Nd2S3 composed of a two neodymium atoms in the +3 oxidation state and three sulfur atoms in the -2 oxidation state. Like other rare earth sulfides, neodymium(III) sulfide is used as a high-performance inorganic pigment.

Europium compounds are compounds formed by the lanthanide metal europium (Eu). In these compounds, europium generally exhibits the +3 oxidation state, such as EuCl3, Eu(NO3)3 and Eu(CH3COO)3. Compounds with europium in the +2 oxidation state are also known. The +2 ion of europium is the most stable divalent ion of lanthanide metals in aqueous solution. Many europium compounds fluoresce under ultraviolet light due to the excitation of electrons to higher energy levels. Lipophilic europium complexes often feature acetylacetonate-like ligands, e.g., Eufod.
Lanthanum(III) sulfide is a binary inorganic chemical compound of lanthanum metal and sulfur with the chemical formula La2S3.
Ammonium hexachloropalladate is an inorganic chemical compound with the chemical formula (NH4)2PdCl6.













