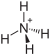 2 2 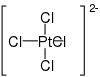 | |
| Names | |
|---|---|
| IUPAC name diazanium;tetrachloroplatinum(2-) | |
| Other names Ammonium tetrachloroplatinate(II) | |
| Identifiers | |
3D model (JSmol) | |
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.034.076 |
| EC Number |
|
| 79515 | |
PubChem CID | |
| UNII | |
CompTox Dashboard (EPA) | |
| |
| |
| Properties | |
| Cl4H8N2Pt | |
| Molar mass | 372.96 g·mol−1 |
| Appearance | red crystals |
| Density | 2,94 g/cm3 |
| Melting point | 140 °C |
| soluble | |
| Hazards | |
| GHS labelling: [1] | |
   | |
| Danger | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Diammonium tetrachloroplatinate is a chemical compound with the chemical formula (NH4)2[PtCl4]. [2] [3]