
Bromine is a chemical element; it has symbol Br and atomic number 35. It is a volatile red-brown liquid at room temperature that evaporates readily to form a similarly coloured vapour. Its properties are intermediate between those of chlorine and iodine. Isolated independently by two chemists, Carl Jacob Löwig and Antoine Jérôme Balard, its name was derived from Ancient Greek βρῶμος (bromos) 'stench', referring to its sharp and pungent smell.

Chlorine is a chemical element; it has symbol Cl and atomic number 17. The second-lightest of the halogens, it appears between fluorine and bromine in the periodic table and its properties are mostly intermediate between them. Chlorine is a yellow-green gas at room temperature. It is an extremely reactive element and a strong oxidising agent: among the elements, it has the highest electron affinity and the third-highest electronegativity on the revised Pauling scale, behind only oxygen and fluorine.

In chemistry, a salt or ionic compound is a chemical compound consisting of an assembly of positively charged ions (cations) and negatively charged ions (anions), which results in a compound with no net electric charge. The constituent ions are held together by electrostatic forces termed ionic bonds.

Potassium chlorate is the inorganic compound with the molecular formula KClO3. In its pure form, it is a white solid. After sodium chlorate, it is the second most common chlorate in industrial use. It is a strong oxidizing agent and its most important application is in safety matches. In other applications it is mostly obsolete and has been replaced by safer alternatives in recent decades. It has been used

Dinitrogen pentoxide is the chemical compound with the formula N2O5. It is one of the binary nitrogen oxides, a family of compounds that contain only nitrogen and oxygen. It exists as colourless crystals that sublime slightly above room temperature, yielding a colorless gas.

Lead(II) chloride (PbCl2) is an inorganic compound which is a white solid under ambient conditions. It is poorly soluble in water. Lead(II) chloride is one of the most important lead-based reagents. It also occurs naturally in the form of the mineral cotunnite.

Sodium perchlorate is an inorganic compound with the chemical formula NaClO4. It consists of sodium cations Na+ and perchlorate anions ClO−4. It is a white crystalline, hygroscopic solid that is highly soluble in water and ethanol. It is usually encountered as sodium perchlorate monohydrate NaClO4·H2O. The compound is noteworthy as the most water-soluble of the common perchlorate salts.
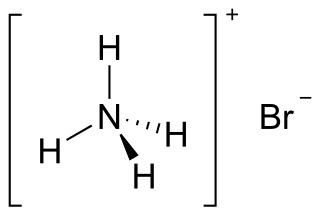
Ammonium bromide, NH4Br, is the ammonium salt of hydrobromic acid. The chemical crystallizes in colorless prisms, possessing a saline taste; it sublimes on heating and is easily soluble in water. On exposure to air it gradually assumes a yellow color because of the oxidation of bromide (Br−) to bromine (Br2).
Selenic acid is the inorganic compound with the formula H2SeO4. It is an oxoacid of selenium, and its structure is more accurately described as O2Se(OH)2. It is a colorless compound. Although it has few uses, one of its salts, sodium selenate is used in the production of glass and animal feeds.

Yttrium(III) chloride is an inorganic compound of yttrium and chloride. It exists in two forms, the hydrate (YCl3(H2O)6) and an anhydrous form (YCl3). Both are colourless salts that are highly soluble in water and deliquescent.

Terbium(III) bromide (TbBr3) is a crystalline chemical compound.
Sodium atoms have 11 electrons, one more than the stable configuration of the noble gas neon. As a result, sodium usually forms ionic compounds involving the Na+ cation. Sodium is a reactive alkali metal and is much more stable in ionic compounds. It can also form intermetallic compounds and organosodium compounds. Sodium compounds are often soluble in water.
Bromine compounds are compounds containing the element bromine (Br). These compounds usually form the -1, +1, +3 and +5 oxidation states. Bromine is intermediate in reactivity between chlorine and iodine, and is one of the most reactive elements. Bond energies to bromine tend to be lower than those to chlorine but higher than those to iodine, and bromine is a weaker oxidising agent than chlorine but a stronger one than iodine. This can be seen from the standard electrode potentials of the X2/X− couples (F, +2.866 V; Cl, +1.395 V; Br, +1.087 V; I, +0.615 V; At, approximately +0.3 V). Bromination often leads to higher oxidation states than iodination but lower or equal oxidation states to chlorination. Bromine tends to react with compounds including M–M, M–H, or M–C bonds to form M–Br bonds.

Barium chlorate, Ba(ClO3)2, is the barium salt of chloric acid. It is a white crystalline solid, and like all soluble barium compounds, irritant and toxic. It is sometimes used in pyrotechnics to produce a green colour. It also finds use in the production of chloric acid.

Bromous acid is the inorganic compound with the formula of HBrO2. It is an unstable compound, although salts of its conjugate base – bromites – have been isolated. In acidic solution, bromites decompose to bromine.
Barium permanganate is a chemical compound, with the formula Ba(MnO4)2. It forms violet to brown crystals that are sparingly soluble in water.

Caesium ozonide is an oxygen-rich chemical compound of caesium, with the chemical formula CsO3. It consists of caesium cations Cs+ and ozonide anions O−3. It can be formed by reacting ozone with caesium superoxide:

Neodymium compounds are compounds formed by the lanthanide metal neodymium (Nd). In these compounds, neodymium generally exhibits the +3 oxidation state, such as NdCl3, Nd2(SO4)3 and Nd(CH3COO)3. Compounds with neodymium in the +2 oxidation state are also known, such as NdCl2 and NdI2. Some neodymium compounds have colors that vary based upon the type of lighting.
Rhenium compounds are compounds formed by the transition metal rhenium (Re). Rhenium can form in many oxidation states, and compounds are known for every oxidation state from -3 to +7 except -2, although the oxidation states +7, +4, and +3 are the most common. Rhenium is most available commercially as salts of perrhenate, including sodium and ammonium perrhenates. These are white, water-soluble compounds. The tetrathioperrhenate anion [ReS4]− is possible.

Ammonium hexafluorogallate is an inorganic chemical compound with the chemical formula (NH4)3GaF6.
















