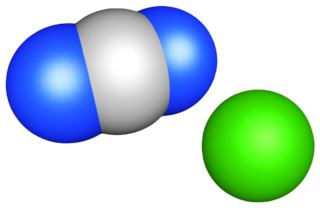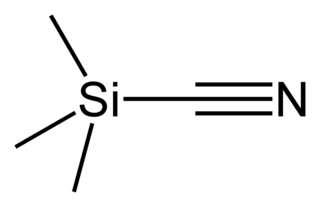Nitrogen is a chemical element; it has symbol N and atomic number 7. Nitrogen is a nonmetal and the lightest member of group 15 of the periodic table, often called the pnictogens. It is a common element in the universe, estimated at seventh in total abundance in the Milky Way and the Solar System. At standard temperature and pressure, two atoms of the element bond to form N2, a colorless and odorless diatomic gas. N2 forms about 78% of Earth's atmosphere, making it the most abundant chemical species in air. Because of the volatility of nitrogen compounds, nitrogen is relatively rare in the solid parts of the Earth.
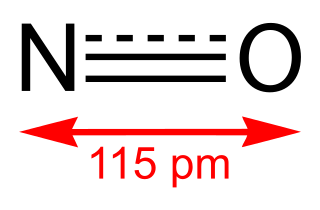
Nitric oxide is a colorless gas with the formula NO. It is one of the principal oxides of nitrogen. Nitric oxide is a free radical: it has an unpaired electron, which is sometimes denoted by a dot in its chemical formula. Nitric oxide is also a heteronuclear diatomic molecule, a class of molecules whose study spawned early modern theories of chemical bonding.

Dinitrogen pentoxide is the chemical compound with the formula N2O5. It is one of the binary nitrogen oxides, a family of compounds that only contain nitrogen and oxygen. It exists as colourless crystals that sublime slightly above room temperature, yielding a colorless gas.
In organic chemistry, a nitrile is any organic compound that has a −C≡N functional group. The name of the compound is composed of a base, which includes the carbon of the −C≡N, suffixed with "nitrile", so for example CH3CH2C≡N is called "propionitrile". The prefix cyano- is used interchangeably with the term nitrile in industrial literature. Nitriles are found in many useful compounds, including methyl cyanoacrylate, used in super glue, and nitrile rubber, a nitrile-containing polymer used in latex-free laboratory and medical gloves. Nitrile rubber is also widely used as automotive and other seals since it is resistant to fuels and oils. Organic compounds containing multiple nitrile groups are known as cyanocarbons.
An isocyanide is an organic compound with the functional group –N+≡C−. It is the isomer of the related nitrile (–C≡N), hence the prefix is isocyano. The organic fragment is connected to the isocyanide group through the nitrogen atom, not via the carbon. They are used as building blocks for the synthesis of other compounds.

Phosphorus pentachloride is the chemical compound with the formula PCl5. It is one of the most important phosphorus chlorides/oxychlorides, others being PCl3 and POCl3. PCl5 finds use as a chlorinating reagent. It is a colourless, water-sensitive solid, although commercial samples can be yellowish and contaminated with hydrogen chloride.

Organotin chemistry is the scientific study of the synthesis and properties of organotin compounds or stannanes, which are organometallic compounds containing tin–carbon bonds. The first organotin compound was diethyltin diiodide, discovered by Edward Frankland in 1849. The area grew rapidly in the 1900s, especially after the discovery of the Grignard reagents, which are useful for producing Sn–C bonds. The area remains rich with many applications in industry and continuing activity in the research laboratory.

Gold(III) chloride, traditionally called auric chloride, is an inorganic compound of gold and chlorine with the molecular formula Au2Cl6. The "III" in the name indicates that the gold has an oxidation state of +3, typical for many gold compounds. It has two forms, the monohydrate (AuCl3·H2O) and the anhydrous form, which are both hygroscopic and light-sensitive solids. This compound is a dimer of AuCl3. This compound has a few uses, such as an oxidizing agent and for catalyzing various organic reactions.

In organic chemistry, a carbodiimide is a functional group with the formula RN=C=NR. On Earth they are exclusively synthetic, but in interstellar space the parent compound HN=C=NH has been detected by its maser emissions.
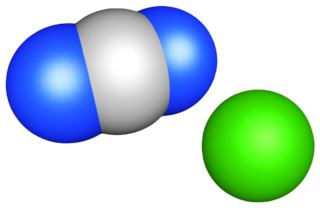
Calcium cyanamide, also known as Calcium carbondiamide, Calcium cyan-2°-amide or Calcium cyanonitride is the inorganic compound with the formula CaCN2. It is the calcium salt of the cyanamide (CN2−
2) anion. This chemical is used as fertilizer and is commercially known as nitrolime. It also has herbicidal activity and in the 1950s was marketed as cyanamid. It was first synthesized in 1898 by Adolph Frank and Nikodem Caro (Frank–Caro process).
Nitryl fluoride, NO2F, is a colourless gas and strong oxidizing agent, which is used as a fluorinating agent and has been proposed as an oxidiser in rocket propellants (though never flown).
Cyanogen bromide is the inorganic compound with the formula (CN)Br or BrCN. It is a colorless solid that is widely used to modify biopolymers, fragment proteins and peptides, and synthesize other compounds. The compound is classified as a pseudohalogen.

In organic chemistry, nitroso refers to a functional group in which the nitric oxide group is attached to an organic moiety. As such, various nitroso groups can be categorized as C-nitroso compounds, S-nitroso compounds, N-nitroso compounds, and O-nitroso compounds.
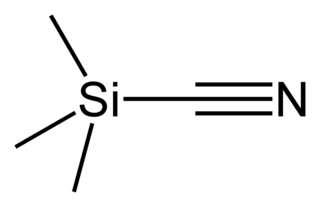
Trimethylsilyl cyanide is the chemical compound with the formula (CH3)3SiCN. This volatile liquid consists of a cyanide group, that is CN, attached to a trimethylsilyl group. The molecule is used in organic synthesis as the equivalent of hydrogen cyanide. It is prepared by the reaction of lithium cyanide and trimethylsilyl chloride:
The chemical element nitrogen is one of the most abundant elements in the universe and can form many compounds. It can take several oxidation states; but the most common oxidation states are -3 and +3. Nitrogen can form nitride and nitrate ions. It also forms a part of nitric acid and nitrate salts. Nitrogen compounds also have an important role in organic chemistry, as nitrogen is part of proteins, amino acids and adenosine triphosphate.

Metal nitrosyl complexes are complexes that contain nitric oxide, NO, bonded to a transition metal. Many kinds of nitrosyl complexes are known, which vary both in structure and coligand.

Nitrosyl chloride is the chemical compound with the formula NOCl. It is a yellow gas that is commonly encountered as a component of aqua regia, a mixture of 3 parts concentrated hydrochloric acid and 1 part of concentrated nitric acid. It is a strong electrophile and oxidizing agent. It is sometimes called Tilden's reagent, after William A. Tilden, who was the first to produce it as a pure compound.
The Barton reaction, also known as the Barton nitrite ester reaction, is a photochemical reaction that involves the photolysis of an alkyl nitrite to form a δ-nitroso alcohol.

Calcium cyanide is the inorganic compound with the formula Ca(CN)2. It is the calcium salt derived from hydrocyanic acid. It is a white solid, although the pure material is rarely encountered. It hydrolyses readily (even in moist air) to release hydrogen cyanide and is very toxic.
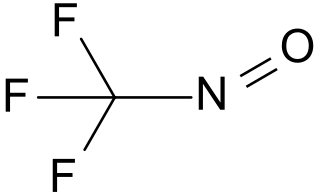
Trifluoronitrosomethane is a toxic organic compound consisting of a trifluoromethyl group covalently bound to a nitroso group. Its distinctive deep blue color is unusual for a gas.