| |
 | |
| Names | |
|---|---|
| IUPAC name Acrylic acid [2] | |
| Preferred IUPAC name 2-Propenoic acid [2] | |
Other names
| |
| Identifiers | |
3D model (JSmol) | |
| 635743 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.001.071 |
| EC Number |
|
| 1817 | |
| KEGG | |
PubChem CID | |
| RTECS number |
|
| UNII | |
| UN number | 2218 |
CompTox Dashboard (EPA) | |
| |
| |
| Properties | |
| C3H4O2 | |
| Molar mass | 72.063 g/mol |
| Appearance | Clear, colorless liquid |
| Odor | Acrid [3] |
| Density | 1.051 g/mL |
| Melting point | 14 °C (57 °F; 287 K) |
| Boiling point | 141 °C (286 °F; 414 K) |
| Miscible | |
| log P | 0.28 [4] |
| Vapor pressure | 3 mmHg [3] |
| Acidity (pKa) | 4.25 (H2O) [5] |
| Viscosity | 1.3 cP at 20 °C (68 °F) |
| Hazards | |
| GHS labelling: | |
     | |
| Danger | |
| H226, H302, H312, H314, H332, H400 | |
| P210, P233, P240, P241, P242, P243, P260, P261, P264, P270, P271, P273, P280, P301+P312, P301+P330+P331, P302+P352, P303+P361+P353, P304+P312, P304+P340, P305+P351+P338, P310, P312, P321, P322, P330, P363, P370+P378, P391, P403+P235, P405, P501 | |
| NFPA 704 (fire diamond) | |
| Flash point | 49.4 [6] °C (120.9 °F; 322.5 K) |
| 429 °C (804 °F; 702 K) | |
| Explosive limits | 2.4–8.02% [3] |
| NIOSH (US health exposure limits): | |
PEL (Permissible) | None [3] |
REL (Recommended) | TWA 2 ppm (6 mg/m3) [skin] [3] |
IDLH (Immediate danger) | N.D. [3] |
| Safety data sheet (SDS) | MSDS |
| Related compounds | |
Other anions | acrylate |
Related carboxylic acids | acetic acid propionic acid lactic acid 3-hydroxypropionic acid malonic acid butyric acid crotonic acid |
Related compounds | allyl alcohol propionaldehyde acrolein methyl acrylate |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
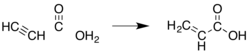
Acrylic acid (IUPAC: prop-2-enoic acid) is an organic compound with the formula CH2=CHCOOH. It is the simplest unsaturated carboxylic acid, consisting of a vinyl group connected directly to a carboxylic acid terminus. This colorless liquid has a characteristic acrid or tart smell. It is miscible with water, alcohols, ethers, and chloroform. More than a million tons are produced annually. [7]