Acrylates are the salts, esters, and conjugate bases of acrylic acid. The acrylate ion is the anion CH2=CHCO−2. Often, acrylate refers to esters of acrylic acid, the most common member being methyl acrylate. These acrylates contain vinyl groups. These compounds are of interest because they are bifunctional: the vinyl group is susceptible to polymerization and the carboxylate group carries myriad functionalities.
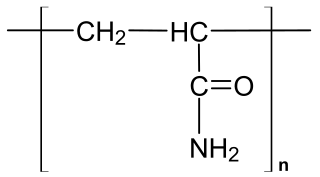
Polyacrylamide (abbreviated as PAM or pAAM) is a polymer with the formula (-CH2CHCONH2-). It has a linear-chain structure. PAM is highly water-absorbent, forming a soft gel when hydrated. In 2008, an estimated 750,000,000 kg were produced, mainly for water treatment and the paper and mineral industries.

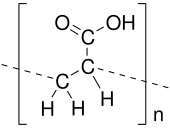
Acrylic acid (IUPAC: propenoic acid) is an organic compound with the formula CH2=CHCOOH. It is the simplest unsaturated carboxylic acid, consisting of a vinyl group connected directly to a carboxylic acid terminus. This colorless liquid has a characteristic acrid or tart smell. It is miscible with water, alcohols, ethers, and chloroform. More than a million tons are produced annually.

Polyelectrolytes are polymers whose repeating units bear an electrolyte group. Polycations and polyanions are polyelectrolytes. These groups dissociate in aqueous solutions (water), making the polymers charged. Polyelectrolyte properties are thus similar to both electrolytes (salts) and polymers and are sometimes called polysalts. Like salts, their solutions are electrically conductive. Like polymers, their solutions are often viscous. Charged molecular chains, commonly present in soft matter systems, play a fundamental role in determining structure, stability and the interactions of various molecular assemblies. Theoretical approaches to describing their statistical properties differ profoundly from those of their electrically neutral counterparts, while technological and industrial fields exploit their unique properties. Many biological molecules are polyelectrolytes. For instance, polypeptides, glycosaminoglycans, and DNA are polyelectrolytes. Both natural and synthetic polyelectrolytes are used in a variety of industries.

Sodium polyacrylate (ACR, ASAP, or PAAS), also known as waterlock, is a sodium salt of polyacrylic acid with the chemical formula [−CH2−CH(CO2Na)−]n and has broad applications in consumer products. This super-absorbent polymer (SAP) has the ability to absorb 100 to 1000 times its mass in water. Sodium polyacrylate is an anionic polyelectrolyte with negatively charged carboxylic groups in the main chain. It is a polymer made up of chains of acrylate compounds. It contains sodium, which gives it the ability to absorb large amounts of water. When dissolved in water, it forms a thick and transparent solution due to the ionic interactions of the molecules. Sodium polyacrylate has many favorable mechanical properties. Some of these advantages include good mechanical stability, high heat resistance, and strong hydration. It has been used as an additive for food products including bread, juice, and ice cream.
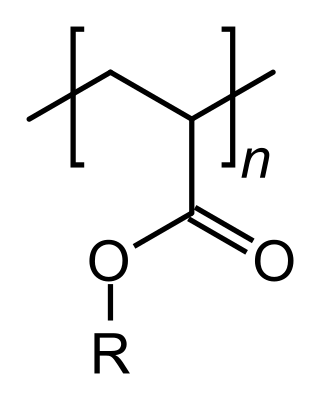
An acrylate polymer is any of a group of polymers prepared from acrylate monomers. These plastics are noted for their transparency, resistance to breakage, and elasticity.

A superabsorbent polymer (SAP) (also called slush powder) is a water-absorbing hydrophilic homopolymers or copolymers that can absorb and retain extremely large amounts of a liquid relative to its own mass.
Solution polymerization is a method of industrial polymerization. In this procedure, a monomer is dissolved in a non-reactive solvent that contains a catalyst or initiator.
Poly(N-isopropylacrylamide) (variously abbreviated PNIPA, PNIPAM, PNIPAAm, NIPA, PNIPAA or PNIPAm) is a temperature-responsive polymer that was first synthesized in the 1950s. It can be synthesized from N-isopropylacrylamide which is commercially available. It is synthesized via free-radical polymerization and is readily functionalized making it useful in a variety of applications.

Temperature-responsive polymers or thermoresponsive polymers are polymers that exhibit drastic and discontinuous changes in their physical properties with temperature. The term is commonly used when the property concerned is solubility in a given solvent, but it may also be used when other properties are affected. Thermoresponsive polymers belong to the class of stimuli-responsive materials, in contrast to temperature-sensitive materials, which change their properties continuously with environmental conditions. In a stricter sense, thermoresponsive polymers display a miscibility gap in their temperature-composition diagram. Depending on whether the miscibility gap is found at high or low temperatures, either an upper critical solution temperature (UCST) or a lower critical solution temperature (LCST) exists.

N,N′-Methylenebisacrylamide (MBAm or MBAA, colloquially "bis") is the organic compound with the formula CH2[NHC(O)CH=CH2]2. A colorless solid, this compound is a crosslinking agent in polyacrylamides, e.g., as used for SDS-PAGE.

2-Acrylamido-2-methylpropane sulfonic acid (AMPS) was a Trademark name by The Lubrizol Corporation. It is a reactive, hydrophilic, sulfonic acid acrylic monomer used to alter the chemical properties of wide variety of anionic polymers. In the 1970s, the earliest patents using this monomer were filed for acrylic fiber manufacturing. Today, there are over several thousands patents and publications involving use of AMPS in many areas including water treatment, oil field, construction chemicals, hydrogels for medical applications, personal care products, emulsion coatings, adhesives, and rheology modifiers.
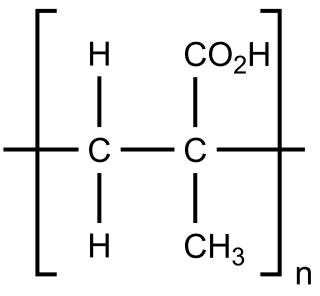
Poly(methacrylic acid) (PMAA) is a polymer made from methacrylic acid (preferred IUPAC name, 2-methylprop-2-enoic acid), which is a carboxylic acid. It is often available as its sodium salt, poly(methacrylic acid) sodium salt. The monomer is a viscous liquid with a pungent odour. The first polymeric form of methacrylic acid was described in 1880 by Engelhorn and Fittig. The use of high purity monomers is required for proper polymerization conditions and therefore it is necessary to remove any inhibitors by extraction (phenolic inhibitors) or via distillation. To prevent inhibition by dissolved oxygen, monomers should be carefully degassed prior to the start of the polymerization.
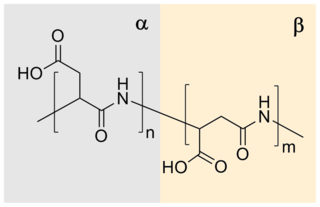
Polyaspartic acid (PASA) is a biodegradable, water-soluble condensation polymer based on the amino acid aspartic acid. It is a biodegradable replacement for water softeners and related applications. PASA can be chemically crosslinked with a wide variety of methods to yield PASA hydrogels. The resulting hydrogels are pH-sensitive such that under acidic conditions, they shrink, while the swelling capacity increases under alkaline conditions.

Vinylsulfonic acid is the organosulfur compound with the chemical formula CH2=CHSO3H. It is the simplest unsaturated sulfonic acid. The C=C double bond is a site of high reactivity. Polymerization gives polyvinylsulfonic acid, especially when used as a comonomer with functionalized vinyl and (meth)acrylic acid compounds. It is a colorless, water-soluble liquid, although commercial samples can appear yellow or even red.
Common hydrogel agriculture's ingredient is potassium polyacrylate or sodium polyacrylate. As a superabsorbent material, it can absorb plenty of water and turn water to gel to store water.
1-Vinylimidazole is a water-soluble basic monomer that forms quaternizable homopolymers by free-radical polymerization with a variety of vinyl and acrylic monomers. The products are functional copolymers, which are used as oil field chemicals and as cosmetic auxiliaries. 1-Vinylimidazole acts as a reactive diluent in UV lacquers, inks, and adhesives.

Potassium polyacrylate is a potassium salt of polyacrylic acid with the chemical formula [−CH2−CH(CO2K)−]n. As a type of superabsorbent polymer, it can absorb hundreds of times its original weight in purified water.
Polysuccinimide (PSI), also known as polyanhydroaspartic acid or polyaspartimide, is formed during the thermal polycondensation of aspartic acid and is the simplest polyimide. Polysuccinimide is insoluble in water, but soluble in some aprotic dipolar solvents. Its reactive nature makes polysuccinimide a versatile starting material for functional polymers made from renewable resources.
Chitosan-poly is a composite that has been increasingly used to create chitosan-poly(acrylic acid) nanoparticles. More recently, various composite forms have come out with poly(acrylic acid) being synthesized with chitosan which is often used in a variety of drug delivery processes. Chitosan which already features strong biodegradability and biocompatibility nature can be merged with polyacrylic acid to create hybrid nanoparticles that allow for greater adhesion qualities as well as promote the biocompatibility and homeostasis nature of chitosan poly(acrylic acid) complex. The synthesis of this material is essential in various applications and can allow for the creation of nanoparticles to facilitate a variety of dispersal and release behaviors and its ability to encapsulate a multitude of various drugs and particles.