
A polymer is a substance or material consisting of very large molecules linked together into chains of repeating subunits. Due to their broad spectrum of properties, both synthetic and natural polymers play essential and ubiquitous roles in everyday life. Polymers range from familiar synthetic plastics such as polystyrene to natural biopolymers such as DNA and proteins that are fundamental to biological structure and function. Polymers, both natural and synthetic, are created via polymerization of many small molecules, known as monomers. Their consequently large molecular mass, relative to small molecule compounds, produces unique physical properties including toughness, high elasticity, viscoelasticity, and a tendency to form amorphous and semicrystalline structures rather than crystals.

Petrochemicals are the chemical products obtained from petroleum by refining. Some chemical compounds made from petroleum are also obtained from other fossil fuels, such as coal or natural gas, or renewable sources such as maize, palm fruit or sugar cane.

Polyethylene or polythene (abbreviated PE; IUPAC name polyethene or poly(methylene)) is the most commonly produced plastic. It is a polymer, primarily used for packaging (plastic bags, plastic films, geomembranes and containers including bottles, etc.). As of 2017, over 100 million tonnes of polyethylene resins are being produced annually, accounting for 34% of the total plastics market.

Polystyrene (PS) is a synthetic polymer made from monomers of the aromatic hydrocarbon styrene. Polystyrene can be solid or foamed. General-purpose polystyrene is clear, hard, and brittle. It is an inexpensive resin per unit weight. It is a poor barrier to air and water vapor and has a relatively low melting point. Polystyrene is one of the most widely used plastics, with the scale of its production being several million tonnes per year. Polystyrene is naturally transparent, but can be colored with colorants. Uses include protective packaging, containers, lids, bottles, trays, tumblers, disposable cutlery, in the making of models, and as an alternative material for phonograph records.

A thermoplastic, or thermosoftening plastic, is any plastic polymer material that becomes pliable or moldable at a certain elevated temperature and solidifies upon cooling.

Acrylonitrile butadiene styrene (ABS) (chemical formula (C8H8)x·(C4H6)y·(C3H3N)z ) is a common thermoplastic polymer. Its glass transition temperature is approximately 105 °C (221 °F). ABS is amorphous and therefore has no true melting point.

Polyacrylonitrile (PAN) is a synthetic, semicrystalline organic polymer resin, with the linear formula (CH2CHCN)n. Almost all PAN resins are copolymers with acrylonitrile as the main monomer. PAN is used to produce large variety of products including ultra filtration membranes, hollow fibers for reverse osmosis, fibers for textiles, and oxidized PAN fibers. PAN fibers are the chemical precursor of very high-quality carbon fiber. PAN is first thermally oxidized in air at 230 °C to form an oxidized PAN fiber and then carbonized above 1000 °C in inert atmosphere to make carbon fibers found in a variety of both high-tech and common daily applications such as civil and military aircraft primary and secondary structures, missiles, solid propellant rocket motors, pressure vessels, fishing rods, tennis rackets and bicycle frames. It is a component repeat unit in several important copolymers, such as styrene-acrylonitrile (SAN) and acrylonitrile butadiene styrene (ABS) plastic.

Styrene-butadiene or styrene-butadiene rubber (SBR) describe families of synthetic rubbers derived from styrene and butadiene. These materials have good abrasion resistance and good aging stability when protected by additives. In 2012, more than 5.4 million tonnes of SBR were processed worldwide. About 50% of car tires are made from various types of SBR. The styrene/butadiene ratio influences the properties of the polymer: with high styrene content, the rubbers are harder and less rubbery. SBR is not to be confused with the thermoplastic elastomer, styrene-butadiene block copolymer, although being derived from the same monomers.

In polymer chemistry, a copolymer is a polymer derived from more than one species of monomer. The polymerization of monomers into copolymers is called copolymerization. Copolymers obtained from the copolymerization of two monomer species are sometimes called bipolymers. Those obtained from three and four monomers are called terpolymers and quaterpolymers, respectively. Copolymers can be characterized by a variety of techniques such as NMR spectroscopy and size-exclusion chromatography to determine the molecular size, weight, properties, and composition of the material.

Polybutadiene [butadiene rubber, BR] is a synthetic rubber. It offers high elasticity, high resistance to wear, good strength even without fillers, and excellent abrasion resistance when filled and vulcanized. "Polybutadiene" is a collective name for homopolymers formed from the polymerization of the monomer 1,3-butadiene. The IUPAC refers to polybutadiene as "poly(buta-1,3-diene)". Historically, an early generation of synthetic polybutadiene rubber produced in Germany by Bayer using sodium as a catalyst was known as "Buna rubber". Polybutadiene is typically crosslinked with sulphur, however, it has also been shown that it can be UV cured when bis-benzophenone additives are incorporated into the formulation.
Kraton is the trade name given to a number of high-performance elastomers manufactured by Kraton Polymers, and used as synthetic replacements for rubber. Kraton polymers offer many of the properties of natural rubber, such as flexibility, high traction, and sealing abilities, but with increased resistance to heat, weathering, and chemicals.

Hot-melt adhesive (HMA), also known as hot glue, is a form of thermoplastic adhesive that is commonly sold as solid cylindrical sticks of various diameters designed to be applied using a hot glue gun. The gun uses a continuous-duty heating element to melt the plastic glue, which the user pushes through the gun either with a mechanical trigger mechanism on the gun, or with direct finger pressure. The glue squeezed out of the heated nozzle is initially hot enough to burn and even blister skin. The glue is sticky when hot, and solidifies in a few seconds to one minute. Hot-melt adhesives can also be applied by dipping or spraying, and are popular with hobbyists and crafters both for affixing and as an inexpensive alternative to resin casting.
Thermoplastic elastomers (TPE), sometimes referred to as thermoplastic rubbers (TPR), are a class of copolymers or a physical mix of polymers that consist of materials with both thermoplastic and elastomeric properties.
Rubber toughening is a process in which rubber nanoparticles are interspersed within a polymer matrix to increase the mechanical robustness, or toughness, of the material. By "toughening" a polymer it is meant that the ability of the polymeric substance to absorb energy and plastically deform without fracture is increased. Considering the significant advantages in mechanical properties that rubber toughening offers, most major thermoplastics are available in rubber-toughened versions; for many engineering applications, material toughness is a deciding factor in final material selection.
Styrene maleic anhydride is a synthetic polymer that is built-up of styrene and maleic anhydride monomers. In one copolymer, the monomers can be almost perfectly alternating. but (random) copolymerisation with less than 50% maleic anhydride content is also possible. The polymer is formed by a radical polymerization, using an organic peroxide as the initiator. The main characteristics of SMA copolymer are its transparent appearance, high heat resistance, high dimensional stability, and the specific reactivity of the anhydride groups. The latter feature results in the solubility of SMA in alkaline (water-based) solutions and dispersion.

Cracks can be formed in many different elastomers by ozone attack, and the characteristic form of attack of vulnerable rubbers is known as ozone cracking. The problem was formerly very common, especially in tires, but is now rarely seen in those products owing to preventive measures.
In polymer chemistry, a comonomer refers to a polymerizable precursor to a copolymer aside from the principal monomer. In some cases, only small amounts of a comonomer are employed, in other cases substantial amounts of comonomers are used. Furthermore, in some cases, the comonomers are statistically incorporated within the polymer chain, whereas in other cases, they aggregate. The distribution of comonomers is referred to as the "blockiness" of a copolymer.
INEOS Styrolution is a global styrenics supplier and is headquartered in Germany. It is a subcompany of INEOS and provides styrenics applications for many everyday products across a broad range of industries, including automotive, electronics, household, construction, healthcare, packaging and toys/sports/leisure.

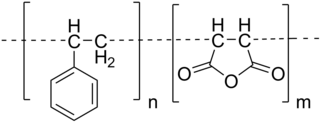
In polymer chemistry, graft polymers are segmented copolymers with a linear backbone of one composite and randomly distributed branches of another composite. The picture labeled "graft polymer" shows how grafted chains of species B are covalently bonded to polymer species A. Although the side chains are structurally distinct from the main chain, the individual grafted chains may be homopolymers or copolymers. Graft polymers have been synthesized for many decades and are especially used as impact resistant materials, thermoplastic elastomers, compatibilizers, or emulsifiers for the preparation of stable blends or alloys. One of the better-known examples of a graft polymer is a component used in high impact polystyrene, consisting of a polystyrene backbone with polybutadiene grafted chains.

Acrylonitrile styrene acrylate (ASA), also called acrylic styrene acrylonitrile, is an amorphous thermoplastic developed as an alternative to acrylonitrile butadiene styrene (ABS), that has improved weather resistance. It is an acrylate rubber-modified styrene acrylonitrile copolymer. It is used for general prototyping in 3D printing, where its UV resistance and mechanical properties make it an excellent material for use in fused filament fabrication printers, particularly for outdoor applications. ASA is also widely used in the automotive industry.

















