Related Research Articles
In polymer chemistry, polymerization, or polymerisation, is a process of reacting monomer molecules together in a chemical reaction to form polymer chains or three-dimensional networks. There are many forms of polymerization and different systems exist to categorize them.
In polymer chemistry, living polymerization is a form of chain growth polymerization where the ability of a growing polymer chain to terminate has been removed. This can be accomplished in a variety of ways. Chain termination and chain transfer reactions are absent and the rate of chain initiation is also much larger than the rate of chain propagation. The result is that the polymer chains grow at a more constant rate than seen in traditional chain polymerization and their lengths remain very similar. Living polymerization is a popular method for synthesizing block copolymers since the polymer can be synthesized in stages, each stage containing a different monomer. Additional advantages are predetermined molar mass and control over end-groups.
Emulsion polymerization is a type of radical polymerization that usually starts with an emulsion incorporating water, monomer, and surfactant. The most common type of emulsion polymerization is an oil-in-water emulsion, in which droplets of monomer are emulsified in a continuous phase of water. Water-soluble polymers, such as certain polyvinyl alcohols or hydroxyethyl celluloses, can also be used to act as emulsifiers/stabilizers. The name "emulsion polymerization" is a misnomer that arises from a historical misconception. Rather than occurring in emulsion droplets, polymerization takes place in the latex/colloid particles that form spontaneously in the first few minutes of the process. These latex particles are typically 100 nm in size, and are made of many individual polymer chains. The particles are prevented from coagulating with each other because each particle is surrounded by the surfactant ('soap'); the charge on the surfactant repels other particles electrostatically. When water-soluble polymers are used as stabilizers instead of soap, the repulsion between particles arises because these water-soluble polymers form a 'hairy layer' around a particle that repels other particles, because pushing particles together would involve compressing these chains.

In materials science, a thermosetting polymer, often called a thermoset, is a polymer that is obtained by irreversibly hardening ("curing") a soft solid or viscous liquid prepolymer (resin). Curing is induced by heat or suitable radiation and may be promoted by high pressure, or mixing with a catalyst. Heat is not necessarily applied externally, but is often generated by the reaction of the resin with a curing agent. Curing results in chemical reactions that create extensive cross-linking between polymer chains to produce an infusible and insoluble polymer network.

Hydroquinone, also known as benzene-1,4-diol or quinol, is an aromatic organic compound that is a type of phenol, a derivative of benzene, having the chemical formula C6H4(OH)2. It has two hydroxyl groups bonded to a benzene ring in a para position. It is a white granular solid. Substituted derivatives of this parent compound are also referred to as hydroquinones. The name "hydroquinone" was coined by Friedrich Wöhler in 1843.
Chain-growth polymerization (AE) or chain-growth polymerisation (BE) is a polymerization technique where unsaturated monomer molecules add onto the active site on a growing polymer chain one at a time. There are a limited number of these active sites at any moment during the polymerization which gives this method its key characteristics.
Free-radical polymerization (FRP) is a method of polymerization, by which a polymer forms by the successive addition of free-radical building blocks. Free radicals can be formed by a number of different mechanisms, usually involving separate initiator molecules. Following its generation, the initiating free radical adds (nonradical) monomer units, thereby growing the polymer chain.

Organic peroxides are organic compounds containing the peroxide functional group (ROOR′). If the R′ is hydrogen, the compounds are called hydroperoxides, which are discussed in that article. Peresters are the peroxy analog of esters and have general structure RC(O)OOR. The O−O bond of peroxides easily breaks, producing free radicals of the form RO•. Thus, organic peroxides are useful as initiators for some types of polymerisation, such as the epoxy resins used in glass-reinforced plastics. MEKP and benzoyl peroxide are commonly used for this purpose. However, the same property also means that organic peroxides can either intentionally or unintentionally initiate explosive polymerisation in materials with unsaturated chemical bonds, and this process has been used in explosives. Organic peroxides, like their inorganic counterparts, are powerful bleaching agents.

Reversible addition−fragmentation chain-transfer or RAFT polymerization is one of several kinds of reversible-deactivation radical polymerization. It makes use of a chain-transfer agent in the form of a thiocarbonylthio compound to afford control over the generated molecular weight and polydispersity during a free-radical polymerization. Discovered at the Commonwealth Scientific and Industrial Research Organisation (CSIRO) of Australia in 1998, RAFT polymerization is one of several living or controlled radical polymerization techniques, others being atom transfer radical polymerization (ATRP) and nitroxide-mediated polymerization (NMP), etc. RAFT polymerization uses thiocarbonylthio compounds, such as dithioesters, thiocarbamates, and xanthates, to mediate the polymerization via a reversible chain-transfer process. As with other controlled radical polymerization techniques, RAFT polymerizations can be performed with conditions to favor low dispersity and a pre-chosen molecular weight. RAFT polymerization can be used to design polymers of complex architectures, such as linear block copolymers, comb-like, star, brush polymers, dendrimers and cross-linked networks.
Autoxidation refers to oxidations brought about by reactions with oxygen at normal temperatures, without the intervention of flame or electric spark. The term is usually used to describe the degradation of organic compounds in air at ambient temperatures. Many common phenomena can be attributed to autoxidation, such as food going rancid, the 'drying' of varnishes and paints, and the perishing of rubber. It is also an important concept in both industrial chemistry and biology. Autoxidation is therefore a fairly broad term and can encompass examples of photooxygenation and catalytic oxidation.
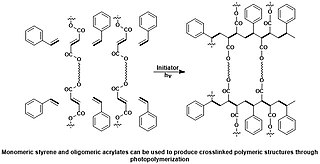
A photopolymer or light-activated resin is a polymer that changes its properties when exposed to light, often in the ultraviolet or visible region of the electromagnetic spectrum. These changes are often manifested structurally, for example hardening of the material occurs as a result of cross-linking when exposed to light. An example is shown below depicting a mixture of monomers, oligomers, and photoinitiators that conform into a hardened polymeric material through a process called curing.

Robert Goulston Gilbert is a polymer chemist whose most significant contributions have been in the field of emulsion polymerisation. In 1970, he gained his PhD from the Australian National University, and worked at the University of Sydney from then until 2006. In 1982, he was elected a fellow of the Royal Australian Chemical Institute; in 1994, he was elected a fellow of the Australian Academy of Science. In 1992, he was appointed full professor, and in 1999 he started the Key Centre for Polymer Colloids, funded by the Australian Research Council, the University and industry. He has served in leadership roles in the International Union of Pure and Applied Chemistry (IUPAC), the world ‘governing body’ of chemistry. He was founding chair (1987–98) of the IUPAC Working Party on the Modelling of Kinetics Processes of Polymerisation, of which he remains a member, and is a member of the IUPAC scientific task groups on starch molecular weight measurements, and terminology. He was vice-president (1996–97) and president (1998–2001) of the IUPAC Macromolecular Division, and secretary of the International Polymer Colloids Group (1997–2001). As of 2007, he is Research Professor at the Centre of Nutrition and Food Science, University of Queensland, where his research program concentrates on the relations between starch structure and nutrition.
Chain transfer is a polymerization reaction by which the activity of a growing polymer chain is transferred to another molecule.

4-tert-Butylcatechol (TBC) is an organic chemical compound which is a derivative of catechol. TBC is available in the form of a solid crystal flake and 85% solution in methanol or water.
Mequinol, MeHQ or 4-methoxyphenol, is a phenol used in dermatology and organic chemistry.
Polymer stabilizers are chemical additives which may be added to polymeric materials, such as plastics and rubbers, to inhibit or retard their degradation. Common polymer degradation processes include oxidation, UV-damage, thermal degradation, ozonolysis, combinations thereof such as photo-oxidation, as well as reactions with catalyst residues, dyes, or impurities. All of these degrade the polymer at a chemical level, via chain scission, uncontrolled recombination and cross-linking, which adversely affects many key properties such as strength, malleability, appearance and colour.
A thermoset polymer matrix is a synthetic polymer reinforcement where polymers act as binder or matrix to secure in place incorporated particulates, fibres or other reinforcements. They were first developed for structural applications, such as glass-reinforced plastic radar domes on aircraft and graphite-epoxy payload bay doors on the space shuttle.
Living free radical polymerization is a type of living polymerization where the active polymer chain end is a free radical. Several methods exist. IUPAC recommends to use the term "reversible-deactivation radical polymerization" instead of "living free radical polymerization", though the two terms are not synonymous.
Plasma polymerization uses plasma sources to generate a gas discharge that provides energy to activate or fragment gaseous or liquid monomer, often containing a vinyl group, in order to initiate polymerization. Polymers formed from this technique are generally highly branched and highly cross-linked, and adhere to solid surfaces well. The biggest advantage to this process is that polymers can be directly attached to a desired surface while the chains are growing, which reduces steps necessary for other coating processes such as grafting. This is very useful for pinhole-free coatings of 100 picometers to 1 micrometre thickness with solvent insoluble polymers.
Aminoxyl denotes a radical functional group with general structure R2N–O•. It is commonly known as a nitroxyl radical or a nitroxide, however IUPAC discourages the use of these terms, as they erroneously suggest the presence of a nitro group. Aminoxyls are structurally related to hydroxylamines and N-oxoammonium salts, with which they can interconvert via a series of redox steps.
References
- ↑ Khuong, Kelli S.; Jones, Walter H.; Pryor, William A.; Houk, K. N. (February 2005). "The Mechanism of the Self-Initiated Thermal Polymerization of Styrene. Theoretical Solution of a Classic Problem". Journal of the American Chemical Society. 127 (4): 1265–1277. doi:10.1021/ja0448667.
- ↑ TUDOS, F; FOLDESBEREZSNICH, T (1989). "Free-radical polymerization: Inhibition and retardation". Progress in Polymer Science. 14 (6): 717–761. doi:10.1016/0079-6700(89)90008-7.
- ↑ Ingold, Keith U. (May 2002). "Peroxy radicals". Accounts of Chemical Research. 2 (1): 1–9. doi:10.1021/ar50013a001.
- ↑ Becker, H.; Vogel, H. (October 2006). "The Role of Hydroquinone Monomethyl Ether in the Stabilization of Acrylic Acid". Chemical Engineering & Technology. 29 (10): 1227–1231. doi:10.1002/ceat.200500401.
- ↑ Pospíšil, Jan; Nešpůrek, Stanislav; Zweifel, Hans (October 1996). "The role of quinone methides in thermostabilization of hydrocarbon polymers —II. Properties and activity mechanisms". Polymer Degradation and Stability. 54 (1): 15–21. doi:10.1016/0141-3910(96)00108-5.
- ↑ Ohkatsu, Yasukazu; Baba, Rie; Watanabe, Keiji (2011). "Radical Scaveging Mechanism of Distearyl Hydroxylamine Antioxidant". Journal of the Japan Petroleum Institute. 54 (1): 15–21. doi: 10.1627/jpi.54.15 .
- ↑ Jackson, R. A.; Waters, William A. (1960). "332. Properties and reactions of free alkyl radicals in solution. Part XIII. Reactions with aromatic nitro-compounds". Journal of the Chemical Society (Resumed): 1653. doi:10.1039/JR9600001653.