Catalysis is the process of increasing the rate of a chemical reaction by adding a substance known as a catalyst. Catalysts are not consumed in the catalyzed reaction but can act repeatedly. Often only very small amounts of catalyst are required. The global demand for catalysts in 2010 was estimated at approximately US$29.5 billion.

Ethers are a class of organic compounds that contain an ether group—an oxygen atom connected to two alkyl or aryl groups. They have the general formula R–O–R′, where R and R′ represent the alkyl or aryl groups. Ethers can again be classified into two varieties: if the alkyl groups are the same on both sides of the oxygen atom, then it is a simple or symmetrical ether, whereas if they are different, the ethers are called mixed or unsymmetrical ethers. A typical example of the first group is the solvent and anesthetic diethyl ether, commonly referred to simply as "ether" (CH3–CH2–O–CH2–CH3). Ethers are common in organic chemistry and even more prevalent in biochemistry, as they are common linkages in carbohydrates and lignin.

A solvent is a substance that dissolves a solute, resulting in a solution. A solvent is usually a liquid but can also be a solid, a gas, or a supercritical fluid. Water is a solvent for polar molecules and the most common solvent used by living things; all the ions and proteins in a cell are dissolved in water within the cell.
Rancidification is the process of complete or incomplete oxidation or hydrolysis of fats and oils when exposed to air, light, or moisture or by bacterial action, resulting in unpleasant taste and odor. Specifically, it is the hydrolysis or autoxidation of fats into short-chain aldehydes, ketones and free fatty acids, which are objectionable in taste and odor. When these processes occur in food, undesirable odors and flavors can result.
In chemistry, reactivity is the impetus for which a chemical substance undergoes a chemical reaction, either by itself or with other materials, with an overall release of energy.

Sodium sulfite (sodium sulphite) is the inorganic compound with the chemical formula Na2SO3. A pale yellow, water-soluble solid, it is used commercially as an antioxidant and preservative. A heptahydrate is also known but it is less useful because of its greater susceptibility toward oxidation by air.

Oxygen scavengers or oxygen absorbers are added to enclosed packaging to help remove or decrease the level of oxygen in the package. They are used to help maintain product safety and extend shelf life. There are many types of oxygen absorbers available to cover a wide array of applications.

Sulfamic acid, also known as amidosulfonic acid, amidosulfuric acid, aminosulfonic acid, and sulfamidic acid, is a molecular compound with the formula H3NSO3. This colourless, water-soluble compound finds many applications. Sulfamic acid melts at 205 °C before decomposing at higher temperatures to water, sulfur trioxide, sulfur dioxide and nitrogen.
A free-radical reaction is any chemical reaction involving free radicals. This reaction type is abundant in organic reactions. Two pioneering studies into free radical reactions have been the discovery of the triphenylmethyl radical by Moses Gomberg (1900) and the lead-mirror experiment described by Friedrich Paneth in 1927. In this last experiment tetramethyllead is decomposed at elevated temperatures to methyl radicals and elemental lead in a quartz tube. The gaseous methyl radicals are moved to another part of the chamber in a carrier gas where they react with lead in a mirror film which slowly disappears.
Chemical fouling inhibitors are products that are mixtures of fouling and corrosion inhibitors use in boiler feedwater treatment. Several of these products use aliphatic polyamines to coat the surface of pipes.
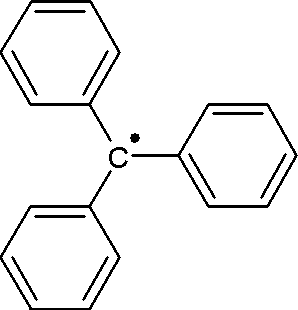
The triphenylmethyl radical is a persistent radical and the first radical ever described in organic chemistry.

A Grignard reagent or Grignard compound is a chemical compound with the generic formula R−Mg−X, where X is a halogen and R is an organic group, normally an alkyl or aryl. Two typical examples are methylmagnesium chloride Cl−Mg−CH
3 and phenylmagnesium bromide (C
6H
5)−Mg−Br. They are a subclass of the organomagnesium compounds.

Trioxidane, also called hydrogen trioxide or dihydrogen trioxide, is an inorganic compound with the chemical formula H[O]
3H. It is one of the unstable hydrogen polyoxides. In aqueous solutions, trioxidane decomposes to form water and singlet oxygen:
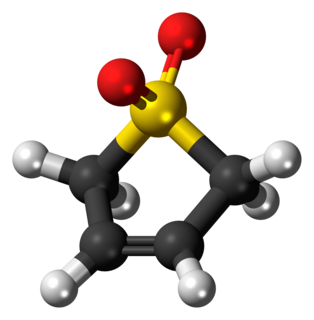
Sulfolene, or butadiene sulfone is a cyclic organic chemical with a sulfone functional group. It is a white, odorless, crystalline, indefinitely storable solid, which dissolves in water and many organic solvents. The compound is used as a source of butadiene.
Polymer stabilizers are chemical additives which may be added to polymeric materials, such as plastics, to inhibit or retard their degradation. Common polymer degradation processes include oxidation, UV-damage, thermal degradation, ozonolysis, combinations thereof such as photo-oxidation, as well as reactions with catalyst residues, dyes, or impurities. All of these degrade the polymer at a chemical level, via chain scission, uncontrolled recombination and cross-linking, which adversely affects many key properties such as strength, malleability, appearance and colour.
Sulfanyl (HS•), also known as the mercapto radical, hydrosulfide radical, or hydridosulfur, is a simple radical molecule consisting of one hydrogen and one sulfur atom. The radical appears in metabolism in organisms as H2S is detoxified. Sulfanyl is one of the top three sulfur-containing gasses in gas giants such as Jupiter and is very likely to be found in brown dwarfs and cool stars. It was originally discovered by Margaret N. Lewis and John U. White at the University of California in 1939. They observed molecular absorption bands around 325 nm belonging to the system designated by 2Σ+ ← 2Πi. They generated the radical by means of a radio frequency discharge in hydrogen sulfide. HS• is formed during the degradation of hydrogen sulfide in the atmosphere of the Earth. This may be a deliberate action to destroy odours or a natural phenomenon.
Cyclopropyl cyanide is an organic compound consisting of a nitrile group as a substituent on a cyclopropane ring. It is the smallest cyclic compound containing a nitrile.
Vanadyl perchlorate or vanadyl triperchlorate is a golden yellow coloured liquid or crystalline compound of vanadium, oxygen and perchlorate group. The substance consists of molecules covalently bound and is quite volatile.

Diethyl oxomalonate is the diethyl ester of mesoxalic acid (ketomalonic acid), the simplest oxodicarboxylic acid and thus the first member (n = 0) of a homologous series HOOC–CO–(CH2)n–COOH with the higher homologues oxalacetic acid (n = 1), α-ketoglutaric acid (n = 2) and α-ketoadipic acid (n = 3) (the latter a metabolite of the amino acid lysine). Diethyl oxomalonate reacts because of its highly polarized keto group as electrophile in addition reactions and is a highly active reactant in pericyclic reactions such as the Diels-Alder reactions, cycloadditions or ene reactions. At humid air, mesoxalic acid diethyl ester reacts with water to give diethyl mesoxalate hydrate and the green-yellow oil are spontaneously converted to white crystals.
Polymerisation inhibitors are chemical compounds added to monomers to prevent their auto-polymerisation. Unsaturated monomers such as acrylates, vinyl chloride, butadiene and styrene require inhibitors for both processing and safe transport and storage. Many monomers are purified industrially by distillation, which can lead to thermally initiated polymerisation. Styrene for example is distilled at temperatures above 100 °C whereupon it undergoes thermal polymerisation at a rate of ~2% per hour. This polymerisation is undesirable, as it can foul the fractionating tower, it is also typically exothermic which can lead to a runaway reaction and potential explosion if left unchecked. Once initiated polymerisation is typically radical in mechanism and as such many polymerisation inhibitors act as radical scavengers.














