
In chemistry, an ester is a functional group derived from an acid in which the hydrogen atom (H) of at least one acidic hydroxyl group of that acid is replaced by an organyl group. Analogues derived from oxygen replaced by other chalcogens belong to the ester category as well. According to some authors, organyl derivatives of acidic hydrogen of other acids are esters as well, but not according to the IUPAC.

Petrochemicals are the chemical products obtained from petroleum by refining. Some chemical compounds made from petroleum are also obtained from other fossil fuels, such as coal or natural gas, or renewable sources such as maize, palm fruit or sugar cane.
Acrylonitrile is an organic compound with the formula CH2CHCN and the structure H2C=CH−C≡N. It is a colorless, volatile liquid. It has a pungent odor of garlic or onions. Its molecular structure consists of a vinyl group linked to a nitrile. It is an important monomer for the manufacture of useful plastics such as polyacrylonitrile. It is reactive and toxic at low doses.
Acrylates are the salts, esters, and conjugate bases of acrylic acid. The acrylate ion is the anion CH2=CHCO−2. Often, acrylate refers to esters of acrylic acid, the most common member being methyl acrylate. These acrylates contain vinyl groups. These compounds are of interest because they are bifunctional: the vinyl group is susceptible to polymerization and the carboxylate group carries myriad functionalities.

Acrylic acid (IUPAC: prop-2-enoic acid) is an organic compound with the formula CH2=CHCOOH. It is the simplest unsaturated carboxylic acid, consisting of a vinyl group connected directly to a carboxylic acid terminus. This colorless liquid has a characteristic acrid or tart smell. It is miscible with water, alcohols, ethers, and chloroform. More than a million tons are produced annually.
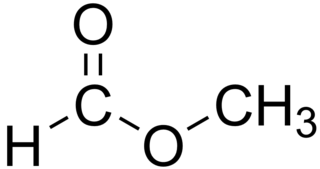
Methyl formate, also called methyl methanoate, is the methyl ester of formic acid. The simplest example of a carboxylate ester, it is a colorless liquid with an ethereal odour, high vapor pressure, and low surface tension. It is a precursor to many other compounds of commercial interest.

Methyl acetate, also known as MeOAc, acetic acid methyl ester or methyl ethanoate, is a carboxylate ester with the formula CH3COOCH3. It is a flammable liquid with a characteristically pleasant smell reminiscent of some glues and nail polish removers. Methyl acetate is occasionally used as a solvent, being weakly polar and lipophilic, but its close relative ethyl acetate is a more common solvent being less toxic and less soluble in water. Methyl acetate has a solubility of 25% in water at room temperature. At elevated temperature its solubility in water is much higher. Methyl acetate is not stable in the presence of strong aqueous bases or aqueous acids. Methyl acetate is not considered a VOC in the USA.

Methyl methacrylate (MMA) is an organic compound with the formula CH2=C(CH3)COOCH3. This colorless liquid, the methyl ester of methacrylic acid (MAA), is a monomer produced on a large scale for the production of poly(methyl methacrylate) (PMMA).

Vinyl acetate is an organic compound with the formula CH3CO2CH=CH2. This colorless liquid is the precursor to polyvinyl acetate, ethene-vinyl acetate copolymers, polyvinyl alcohol, and other important industrial polymers.
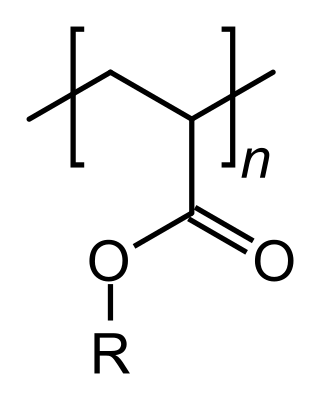
An acrylate polymer is any of a group of polymers prepared from acrylate monomers. These plastics are noted for their transparency, resistance to breakage, and elasticity.
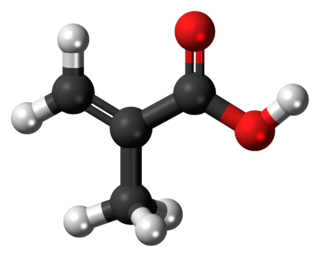
Methacrylic acid, abbreviated MAA, is an organic compound with the formula CH2=C(CH3)CO2H. This colorless, viscous liquid is a carboxylic acid with an acrid unpleasant odor. It is soluble in warm water and miscible with most organic solvents. Methacrylic acid is produced industrially on a large scale as a precursor to its esters, especially methyl methacrylate (MMA), and to poly(methyl methacrylate) (PMMA).

Ethyl acrylate is an organic compound with the formula CH2CHCO2CH2CH3. It is the ethyl ester of acrylic acid. It is a colourless liquid with a characteristic acrid odor. It is mainly produced for paints, textiles, and non-woven fibers. It is also a reagent in the synthesis of various pharmaceutical intermediates.
Solution polymerization is a method of industrial polymerization. In this procedure, a monomer is dissolved in a non-reactive solvent that contains a catalyst or initiator.
In polymer chemistry, a comonomer refers to a polymerizable precursor to a copolymer aside from the principal monomer. In some cases, only small amounts of a comonomer are employed, in other cases substantial amounts of comonomers are used. Furthermore, in some cases, the comonomers are statistically incorporated within the polymer chain, whereas in other cases, they aggregate. The distribution of comonomers is referred to as the "blockiness" of a copolymer.
In organic chemistry, the Baylis–Hillman, Morita–Baylis–Hillman, or MBH reaction is a carbon-carbon bond-forming reaction between an activated alkene and a carbon electrophile in the presence of a nucleophilic catalyst, such as a tertiary amine or phosphine. The product is densely functionalized, joining the alkene at the α-position to a reduced form of the electrophile.
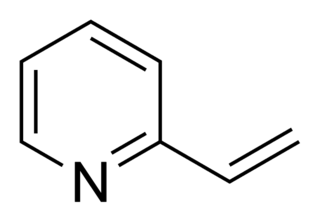
2-Vinylpyridine is an organic compound with the formula CH2CHC5H4N. It is a derivative of pyridine with a vinyl group in the 2-position, next to the nitrogen. It is a colorless liquid, although samples are often brown. It is used industrially as a precursor to specialty polymers and as an intermediate in the chemical, pharmaceutical, dye, and photo industries. Vinylpyridine is sensitive to polymerization. It may be stabilized with a polymerisation inhibitor such as tert-butylcatechol. Owing to its tendency to polymerize, samples are typically refrigerated.
Butyl acrylate is an organic compound with the formula C4H9O2CCH=CH2. A colorless liquid, it is the butyl ester of acrylic acid. It is used commercially on a large scale as a precursor to poly(butyl acrylate). Especially as copolymers, such materials are used in paints, sealants, coatings, adhesives, fuel, textiles, plastics, and caulk.

2-Ethylhexyl acrylate is a colorless liquid acrylate used in the making of paints, plastics and adhesives. It has an odor that has been variously described as pleasant or acrid and musty.
Dimethylaminoethyl acrylate or DMAEA is an unsaturated carboxylic acid ester having a tertiary amino group. It is a colorless to yellowish, water-miscible liquid with a pungent, amine-like odor. DMAEA is an important acrylic monomer that gives basic properties to copolymers.

Hydroxyethyl acrylate is an organic chemical and an aliphatic compound. It has the formula C5H8O3 and the CAS Registry Number 818–61–1. It is REACH registered with an EU number of 212–454–9. It has dual functionality containing a polymerizable acrylic group and a terminal hydroxy group. It is used to make emulsion polymers along with other monomers and the resultant resins are used in coatings, sealants, adhesives and elastomers and other applications.















