Iridium is a chemical element with the symbol Ir and atomic number 77. A very hard, brittle, silvery-white transition metal of the platinum group, it is considered the second-densest naturally occurring metal with a density of 22.56 g/cm3 (0.815 lb/cu in) as defined by experimental X-ray crystallography. It is one of the most corrosion-resistant metals, even at temperatures as high as 2,000 °C (3,630 °F). However, corrosion-resistance is not quantifiable in absolute terms; although only certain molten salts and halogens are corrosive to solid iridium, finely divided iridium dust is much more reactive and can be flammable, whereas gold dust is not flammable but can be attacked by substances that iridium resists, such as aqua regia.

Ruthenium is a chemical element with the symbol Ru and atomic number 44. It is a rare transition metal belonging to the platinum group of the periodic table. Like the other metals of the platinum group, ruthenium is inert to most other chemicals. Russian-born scientist of Baltic-German ancestry Karl Ernst Claus discovered the element in 1844 at Kazan State University and named ruthenium in honor of Russia. Ruthenium is usually found as a minor component of platinum ores; the annual production has risen from about 19 tonnes in 2009 to some 35.5 tonnes in 2017. Most ruthenium produced is used in wear-resistant electrical contacts and thick-film resistors. A minor application for ruthenium is in platinum alloys and as a chemistry catalyst. A new application of ruthenium is as the capping layer for extreme ultraviolet photomasks. Ruthenium is generally found in ores with the other platinum group metals in the Ural Mountains and in North and South America. Small but commercially important quantities are also found in pentlandite extracted from Sudbury, Ontario, and in pyroxenite deposits in South Africa.

The Cativa process is a method for the production of acetic acid by the carbonylation of methanol. The technology, which is similar to the Monsanto process, was developed by BP Chemicals and is under license by BP Plc. The process is based on an iridium-containing catalyst, such as the complex [Ir(CO)2I2]− (1).

Aluminium iodide is a chemical compound containing aluminium and iodine. Invariably, the name refers to a compound of the composition AlI
3, formed by the reaction of aluminium and iodine or the action of HI on Al metal. The hexahydrate is obtained from a reaction between metallic aluminum or aluminum hydroxide with hydrogen iodide or hydroiodic acid. Like the related chloride and bromide, AlI
3 is a strong Lewis acid and will absorb water from the atmosphere. It is employed as a reagent for the scission of certain kinds of C-O and N-O bonds. It cleaves aryl ethers and deoxygenates epoxides.

Rubidium iodide is a salt of rubidium and iodine, with the chemical formula RbI. It is a white solid with a melting point of 642 °C.

Bismuth(III) iodide is the inorganic compound with the formula BiI3. This gray-black salt is the product of the reaction of bismuth and iodine, which once was of interest in qualitative inorganic analysis.

Organoiridium chemistry is the chemistry of organometallic compounds containing an iridium-carbon chemical bond. Organoiridium compounds are relevant to many important processes including olefin hydrogenation and the industrial synthesis of acetic acid. They are also of great academic interest because of the diversity of the reactions and their relevance to the synthesis of fine chemicals.

Rhodocene is a chemical compound with the formula [Rh(C5H5)2]. Each molecule contains an atom of rhodium bound between two planar aromatic systems of five carbon atoms known as cyclopentadienyl rings in a sandwich arrangement. It is an organometallic compound as it has (haptic) covalent rhodium–carbon bonds. The [Rh(C5H5)2] radical is found above 150 °C (302 °F) or when trapped by cooling to liquid nitrogen temperatures (−196 °C [−321 °F]). At room temperature, pairs of these radicals join via their cyclopentadienyl rings to form a dimer, a yellow solid.

Germanium(II) iodide is an iodide of germanium, with the chemical formula of GeI2.
Californium(III) chloride is an inorganic compound with a chemical formula CfCl3. Like in californium oxide (Cf2O3) and other californium halides, including californium fluoride (CfF3) and iodide (CfI3), the californium atom has an oxidation state of +3.
Iron(III) iodide is an inorganic compound with the chemical formula FeI3. It is a thermodynamically unstable compound that is difficult to prepare. Nevertheless, iron(III) iodide has been synthesised in small quantities in the absence of air and water.

Praseodymium(III) iodide is an inorganic salt, consisting of the rare-earth metal praseodymium with hydrogen iodide with the chemical formula PrI3. It forms green crystals. It is soluble in water.
Europium(III) iodide is an inorganic compound containing europium and iodine with the chemical formula EuI3.

Gadolinium diiodide is an inorganic compound, with the chemical formula of GdI2. It is an electride, with the ionic formula of Gd3+(I−)2e−, and therefore not a true gadolinium(II) compound. It is ferromagnetic at 276 K with a saturation magnetization of 7.3 B; it exhibits a large negative magnetoresistance (~70%) at 7 T near room temperature. It can be obtained by reacting gadolinium and gadolinium(III) iodide at a high temperature:
Promethium(III) iodide is an inorganic compound, with the chemical formula of PmI3. It is radioactive.

Holmium(III) iodide is an iodide of holmium, with the chemical formula of HoI3. It is used as a component of metal halide lamps.
Lutetium compounds are compounds formed by the lanthanide metal lutetium (Lu). In these compounds, lutetium generally exhibits the +3 oxidation state, such as LuCl3, Lu2O3 and Lu2(SO4)3. Aqueous solutions of most lutetium salts are colorless and form white crystalline solids upon drying, with the common exception of the iodide. The soluble salts, such as nitrate, sulfate and acetate form hydrates upon crystallization. The oxide, hydroxide, fluoride, carbonate, phosphate and oxalate are insoluble in water.
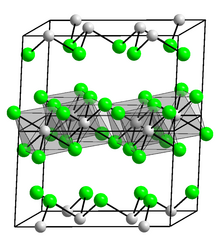
Iridium compounds are compounds containing the element iridium (Ir). Iridium forms compounds in oxidation states between −3 and +9, but the most common oxidation states are +1, +3, and +4. Well-characterized compounds containing iridium in the +6 oxidation state include IrF6 and the oxides Sr2MgIrO6 and Sr2CaIrO6. iridium(VIII) oxide was generated under matrix isolation conditions at 6 K in argon. The highest oxidation state (+9), which is also the highest recorded for any element, is found in gaseous [IrO4]+.
Ruthenium(III) iodide is a chemical compound containing ruthenium and iodine with the formula RuI3. It is a black solid.
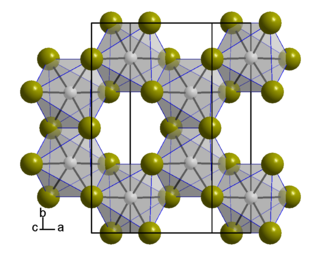
Iridium(III) bromide is a bromide of iridium(III), with the chemical formula of IrBr3.