
Dysprosium is a chemical element; it has symbol Dy and atomic number 66. It is a rare-earth element in the lanthanide series with a metallic silver luster. Dysprosium is never found in nature as a free element, though, like other lanthanides, it is found in various minerals, such as xenotime. Naturally occurring dysprosium is composed of seven isotopes, the most abundant of which is 164Dy.

Ruthenium is a chemical element; it has symbol Ru and atomic number 44. It is a rare transition metal belonging to the platinum group of the periodic table. Like the other metals of the platinum group, ruthenium is unreactive to most other chemicals. Karl Ernst Claus, a Russian-born scientist of Baltic-German ancestry, discovered the element in 1844 at Kazan State University and named ruthenium in honor of Russia. Ruthenium is usually found as a minor component of platinum ores; the annual production has risen from about 19 tonnes in 2009 to some 35.5 tonnes in 2017. Most ruthenium produced is used in wear-resistant electrical contacts and thick-film resistors. A minor application for ruthenium is in platinum alloys and as a chemistry catalyst. A new application of ruthenium is as the capping layer for extreme ultraviolet photomasks. Ruthenium is generally found in ores with the other platinum group metals in the Ural Mountains and in North and South America. Small but commercially important quantities are also found in pentlandite extracted from Sudbury, Ontario, and in pyroxenite deposits in South Africa.

Dysprosium(III) chloride (DyCl3), also known as dysprosium trichloride, is a compound of dysprosium and chlorine. It is a white to yellow solid which rapidly absorbs water on exposure to moist air to form a hexahydrate, DyCl3·6H2O. Simple rapid heating of the hydrate causes partial hydrolysis to an oxychloride, DyOCl.

Ruthenium(III) chloride is the chemical compound with the formula RuCl3. "Ruthenium(III) chloride" more commonly refers to the hydrate RuCl3·xH2O. Both the anhydrous and hydrated species are dark brown or black solids. The hydrate, with a varying proportion of water of crystallization, often approximating to a trihydrate, is a commonly used starting material in ruthenium chemistry.
Ruthenium tetroxide is the inorganic compound with the formula RuO4. It is a yellow volatile solid that melts near room temperature. It has the odor of ozone. Samples are typically black due to impurities. The analogous OsO4 is more widely used and better known. It is also the anhydride of hyperruthenic acid (H2RuO5). One of the few solvents in which RuO4 forms stable solutions is CCl4.
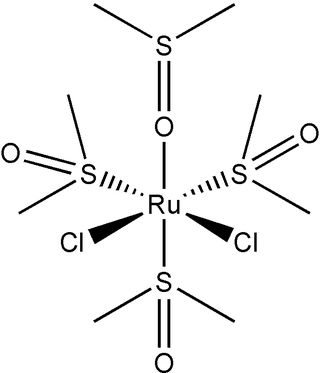
Dichlorotetrakis(dimethyl sulfoxide) ruthenium(II) describes coordination compounds with the formula RuCl2(dmso)4, where DMSO is dimethylsulfoxide. Both cis and trans isomers are known, but the cis isomer is more common. The cis isomer is a yellow, air-stable solid that is soluble in some organic solvents. These sulfoxide complexes are used in the synthesis of various ruthenium(ii) complexes. They have also attracted attention as possible anti-cancer drugs.

Organoruthenium chemistry is the chemistry of organometallic compounds containing a carbon to ruthenium chemical bond. Several organoruthenium catalysts are of commercial interest and organoruthenium compounds have been considered for cancer therapy. The chemistry has some stoichiometric similarities with organoiron chemistry, as iron is directly above ruthenium in group 8 of the periodic table. The most important reagents for the introduction of ruthenium are ruthenium(III) chloride and triruthenium dodecacarbonyl.
Ruthenium hexafluoride, also ruthenium(VI) fluoride (RuF6), is a compound of ruthenium and fluorine and one of the seventeen known binary hexafluorides.
Ruthenium anti-cancer drugs are coordination complexes of ruthenium complexes that have anticancer properties. They promise to provide alternatives to platinum-based drugs for anticancer therapy. No ruthenium anti-cancer drug has been commercialized.

Dysprosium acetylacetonate is a chemical compound of dysprosium with formula Dy(C5H7O2)3(H2O)n.

Ruthenium pentafluoride is the inorganic compound with the empirical formula RuF5. This green volatile solid has rarely been studied but is of interest as a binary fluoride of ruthenium, i.e. a compound containing only Ru and F. It is sensitive toward hydrolysis. Its structure consists of Ru4F20 tetramers, as seen in the isostructural platinum pentafluoride. Within the tetramers, each Ru adopts octahedral molecular geometry, with two bridging fluoride ligands.
Oxyarsenides or arsenide oxides are chemical compounds formally containing the group AsO, with one arsenic and one oxygen atom. The arsenic and oxygen are not bound together as in arsenates or arsenites, instead they make a separate presence bound to the cations (metals), and could be considered as a mixed arsenide-oxide compound. So a compound with OmAsn requires cations to balance a negative charge of 2m+3n. The cations will have charges of +2 or +3. The trications are often rare earth elements or actinides. They are in the category of oxypnictide compounds.
Dysprosium(III) fluoride is an inorganic compound of dysprosium with a chemical formula DyF3.

Ruthenium(III) bromide is a chemical compound of ruthenium and bromine with the formula RuBr3. It is a dark brown solid that decomposes above 400 °C.
Dysprosium(II) chloride (DyCl2), also known as dysprosium dichloride, is an ionic chemical compound of dysprosium and chlorine. This salt is a reduced compound, as the normal oxidation state of dysprosium in dysprosium compounds is +3.
Dysprosium(III) nitrate is an inorganic compound, a salt of dysprosium and nitric acid with the chemical formula Dy(NO3)3. The compound forms yellowish crystals, dissolves in water, forms a crystalline hydrate.

Dysprosium phosphide is an inorganic compound of dysprosium and phosphorus with the chemical formula DyP.

Dysprosium acetate is a hypothetical salt of dysprosium and acetate. Its proposed chemical formula is Dy(CH3COO)3.
Ruthenium(III) iodide is a chemical compound containing ruthenium and iodine with the formula RuI3. It is a black solid.

Dysprosium(III) iodide is a binary inorganic compound of dysprosium and iodine with the chemical formula DyI
3.













