
Boron nitride is a thermally and chemically resistant refractory compound of boron and nitrogen with the chemical formula BN. It exists in various crystalline forms that are isoelectronic to a similarly structured carbon lattice. The hexagonal form corresponding to graphite is the most stable and soft among BN polymorphs, and is therefore used as a lubricant and an additive to cosmetic products. The cubic variety analogous to diamond is called c-BN; it is softer than diamond, but its thermal and chemical stability is superior. The rare wurtzite BN modification is similar to lonsdaleite but slightly softer than the cubic form.

Thulium is a chemical element; it has symbol Tm and atomic number 69. It is the thirteenth and third-last element in the lanthanide series. Like the other lanthanides, the most common oxidation state is +3, seen in its oxide, halides and other compounds; however, the +2 oxidation state can also be stable. In aqueous solution, like compounds of other late lanthanides, soluble thulium compounds form coordination complexes with nine water molecules.

Proton emission is a rare type of radioactive decay in which a proton is ejected from a nucleus. Proton emission can occur from high-lying excited states in a nucleus following a beta decay, in which case the process is known as beta-delayed proton emission, or can occur from the ground state of very proton-rich nuclei, in which case the process is very similar to alpha decay. For a proton to escape a nucleus, the proton separation energy must be negative —the proton is therefore unbound, and tunnels out of the nucleus in a finite time. The rate of proton emission is governed by the nuclear, Coulomb, and centrifugal potentials of the nucleus, where centrifugal potential affects a large part of the rate of proton emission. Half-life of proton emission is affected by the proton energy and its orbital angular momentum. Proton emission is not seen in naturally occurring isotopes; proton emitters can be produced via nuclear reactions, usually using linear particle accelerators.
In chemistry, a nitride is an inorganic compound of nitrogen. The "nitride" anion, N3- ion, is very elusive but compounds of nitride are numerous, although rarely naturally occurring. Some nitrides have a found applications, such as wear-resistant coatings (e.g., titanium nitride, TiN), hard ceramic materials (e.g., silicon nitride, Si3N4), and semiconductors (e.g., gallium nitride, GaN). The development of GaN-based light emitting diodes was recognized by the 2014 Nobel Prize in Physics. Metal nitrido complexes are also common.

Titanium nitride is an extremely hard ceramic material, often used as a physical vapor deposition (PVD) coating on titanium alloys, steel, carbide, and aluminium components to improve the substrate's surface properties.
A blue laser emits electromagnetic radiation with a wavelength between 400 and 500 nanometers, which the human eye sees in the visible spectrum as blue or violet.
Naturally occurring thulium (69Tm) is composed of one stable isotope, 169Tm. Thirty-nine radioisotopes have been characterized, with the most stable being 171Tm with a half-life of 1.92 years, 170Tm with a half-life of 128.6 days, 168Tm with a half-life of 93.1 days, and 167Tm with a half-life of 9.25 days. All of the remaining radioactive isotopes have half-lives that are less than 64 hours, and the majority of these have half-lives that are less than 2 minutes. This element also has 26 meta states, with the most stable being 164mTm, 160mTm and 155mTm.

Thulium(III) oxide is a pale green solid compound, with the formula Tm2O3. It was first isolated in 1879, from an impure sample of erbia, by Swedish chemist Per Teodor Cleve, who named it thulia. It can be prepared in the laboratory by burning thulium metal in air, or by decomposition of their oxoacid salts, such as thulium nitrate.

Silicon nitride is a chemical compound of the elements silicon and nitrogen. Si
3N
4 is the most thermodynamically stable and commercially important of the silicon nitrides, and the term ″Silicon nitride″ commonly refers to this specific composition. It is a white, high-melting-point solid that is relatively chemically inert, being attacked by dilute HF and hot H
3PO
4. It is very hard. It has a high thermal stability with strong optical nonlinearities for all-optical applications.
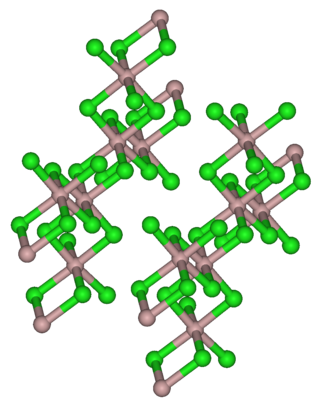
Thulium(III) chloride or thulium trichloride is as an inorganic salt composed of thulium and chlorine with the formula TmCl3. It forms yellow crystals. Thulium(III) chloride has the YCl3 (AlCl3) layer structure with octahedral thulium ions. It has been used as a starting material for some exotic nanostructures prepared for NIR photocatalysis.
Thulium(III) bromide is a crystalline compound of one thulium atom and three bromine atoms. The salt is a white powder at room temperature. It is hygroscopic.
Thulium(II) chloride is an inorganic compound with the chemical formula TmCl2.

Thulium(III) hydroxide is an inorganic compound with the chemical formula Tm(OH)3.

Thulium(III) nitrate is an inorganic compound, a salt of thulium and nitric acid with the chemical formula Tm(NO3)3. The compound forms dark-green crystals, readily soluble in water, also forms crystalline hydrates.
Thulium phosphide is an inorganic compound of thulium and phosphorus with the chemical formula TmP.

Neodymium(II) iodide or neodymium diiodide is an inorganic salt of iodine and neodymium the formula NdI2. Neodymium uses the +2 oxidation state in the compound.
Thulium(II) fluoride is one of the fluoride salts of the lanthanide metal thulium, with the chemical compound of TmF2. It can react with zirconium tetrafluoride at 900 °C to form TmZrF6, which has a hexagonal structure. In addition, low-temperature Mössbauer spectroscopy and some theoretical studies of thulium(II) fluoride have also been reported.

Thulium(III) acetate is the acetate salt of thulium, with the chemical formula of Tm(CH3COO)3. It can exist in the tetrahydrate or the anhydrous form.

Thulium(III) iodide is an iodide of thulium, with the chemical formula of TmI3. Thulium(III) iodide is used as a component of metal halide lamps.
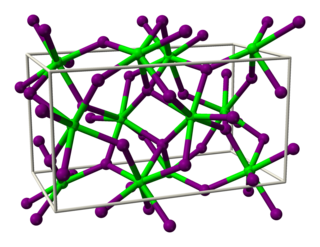
Thulium dibromide is an inorganic compound, with the chemical formula of TmBr2. It is a dark green solid that is easy to dissolve, with the SrI2 structure and it needs to be stored in an inert atmosphere.













