In chemistry, halogenation is a chemical reaction which introduces one or more halogens into a chemical compound. Halide-containing compounds are pervasive, making this type of transformation important, e.g. in the production of polymers, drugs. This kind of conversion is in fact so common that a comprehensive overview is challenging. This article mainly deals with halogenation using elemental halogens. Halides are also commonly introduced using salts of the halides and halogen acids. Many specialized reagents exist for and introducing halogens into diverse substrates, e.g. thionyl chloride.

Acetyl chloride is an acyl chloride derived from acetic acid. It belongs to the class of organic compounds called acid halides. It is a colorless, corrosive, volatile liquid. Its formula is commonly abbreviated to AcCl.

Thionyl chloride is an inorganic compound with the chemical formula SOCl2. It is a moderately volatile, colourless liquid with an unpleasant acrid odour. Thionyl chloride is primarily used as a chlorinating reagent, with approximately 45,000 tonnes per year being produced during the early 1990s, but is occasionally also used as a solvent. It is toxic, reacts with water, and is also listed under the Chemical Weapons Convention as it may be used for the production of chemical weapons.

Phosphorus pentachloride is the chemical compound with the formula PCl5. It is one of the most important phosphorus chlorides/oxychlorides, others being PCl3 and POCl3. PCl5 finds use as a chlorinating reagent. It is a colourless, water-sensitive solid, although commercial samples can be yellowish and contaminated with hydrogen chloride.

Phosphorus trichloride is an inorganic compound with the chemical formula PCl3. A colorless liquid when pure, it is an important industrial chemical, being used for the manufacture of phosphites and other organophosphorus compounds. It is toxic and reacts readily with water to release hydrogen chloride.

Triphenylphosphine (IUPAC name: triphenylphosphane) is a common organophosphorus compound with the formula P(C6H5)3 and often abbreviated to PPh3 or Ph3P. It is versatile compound that is widely used as a reagent in organic synthesis and as a ligand for transition metal complexes, including ones that serve as catalysts in organometallic chemistry. PPh3 exists as relatively air stable, colorless crystals at room temperature. It dissolves in non-polar organic solvents such as benzene and diethyl ether.

Rhodium(III) chloride refers to inorganic compounds with the formula RhCl3(H2O)n, where n varies from 0 to 3. These are diamagnetic red-brown solids. The soluble trihydrated (n = 3) salt is the usual compound of commerce. It is widely used to prepare compounds used in homogeneous catalysis.

Phosphorous acid is the compound described by the formula H3PO3. This acid is diprotic, not triprotic as might be suggested by this formula. Phosphorous acid is an intermediate in the preparation of other phosphorus compounds. Organic derivatives of phosphorous acid, compounds with the formula RPO3H2, are called phosphonic acids.

Phosphoryl chloride is a colourless liquid with the formula POCl3. It hydrolyses in moist air releasing phosphoric acid and fumes of hydrogen chloride. It is manufactured industrially on a large scale from phosphorus trichloride and oxygen or phosphorus pentoxide. It is mainly used to make phosphate esters.

In organic chemistry, a phosphite ester or organophosphite usually refers to an organophosphorous compound with the formula P(OR)3. They can be considered as esters of an unobserved tautomer phosphorous acid, H3PO3, with the simplest example being trimethylphosphite, P(OCH3)3. Some phosphites can be considered esters of the dominant tautomer of phosphorous acid (HP(O)(OH)2). The simplest representative is dimethylphosphite with the formula HP(O)(OCH3)2. Both classes of phosphites are usually colorless liquids.
Boron trichloride is the inorganic compound with the formula BCl3. This colorless gas is a reagent in organic synthesis. It is highly reactive towards water.

Vanadium oxytrichloride is the inorganic compound with the formula VOCl3. This yellow distillable liquid hydrolyzes readily in air. It is an oxidizing agent. It is used as a reagent in organic synthesis. Samples often appear red or orange owing to an impurity of vanadium tetrachloride.
Organophosphorus chemistry is the scientific study of the synthesis and properties of organophosphorus compounds, which are organic compounds containing phosphorus. They are used primarily in pest control as an alternative to chlorinated hydrocarbons that persist in the environment. Some organophosphorus compounds are highly effective insecticides, although some are extremely toxic to humans, including sarin and VX nerve agents.
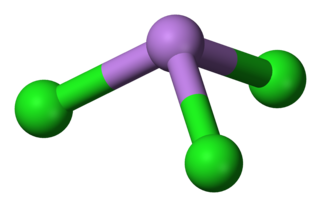
Arsenic trichloride is an inorganic compound with the formula AsCl3, also known as arsenous chloride or butter of arsenic. This poisonous oil is colourless, although impure samples may appear yellow. It is an intermediate in the manufacture of organoarsenic compounds.

Hexachlorophosphazene is an inorganic compound with the chemical formula (NPCl2)3. The molecule has a cyclic, unsaturated backbone consisting of alternating phosphorus and nitrogen atoms, and can be viewed as a trimer of the hypothetical compound N≡PCl2. Its classification as a phosphazene highlights its relationship to benzene. There is large academic interest in the compound relating to the phosphorus-nitrogen bonding and phosphorus reactivity.
Organoarsenic chemistry is the chemistry of compounds containing a chemical bond between arsenic and carbon. A few organoarsenic compounds, also called "organoarsenicals," are produced industrially with uses as insecticides, herbicides, and fungicides. In general these applications are declining in step with growing concerns about their impact on the environment and human health. The parent compounds are arsane and arsenic acid. Despite their toxicity, organoarsenic biomolecules are well known.

In organosulfur chemistry, a sulfenyl chloride is a functional group with the connectivity R−S−Cl, where R is alkyl or aryl. Sulfenyl chlorides are reactive compounds that behave as sources of RS+. They are used in the formation of RS−N and RS−O bonds. According to IUPAC nomenclature they are named as alkyl thiohypochlorites, i.e. esters of thiohypochlorous acid.

Thiophosphoryl fluoride is an inorganic molecular gas with formula PSF3 containing phosphorus, sulfur and fluorine. It spontaneously ignites in air and burns with a cool flame. The discoverers were able to have flames around their hands without discomfort, and called it "probably one of the coldest flames known". The gas was discovered in 1888.

Diethyl phosphite is the organophosphorus compound with the formula (C2H5O)2P(O)H. It is a popular reagent for generating other organophosphorus compounds, exploiting the high reactivity of the P-H bond. Diethyl phosphite is a colorless liquid. The molecule is tetrahedral.
In organophosphorus chemistry, aminophosphines are compounds with the formula R3−nP(NR2)n where R is a hydrogen or organic substituent, and n = 0, 1, or 2. At one extreme, the parents H2PNH2 and P(NH2)3 are lightly studied and fragile. At the other extreme, tris(dimethylamino)phosphine (P(NMe2)3) is commonly available. Intermediate members are known, such as Ph2PN(H)Ph. Aminophosphines are typically colorless and reactive to oxygen. Aminophosphines are pyramidal at the phosphorus.




















