
Bromine is a chemical element with the symbol Br and atomic number 35. It is a volatile red-brown liquid at room temperature that evaporates readily to form a similarly coloured vapour. Its properties are intermediate between those of chlorine and iodine. Isolated independently by two chemists, Carl Jacob Löwig and Antoine Jérôme Balard, its name was derived from the Ancient Greek βρῶμος (bromos) meaning "stench", referring to its sharp and pungent smell.
The haloalkanes are alkanes containing one or more halogen substituents. They are a subset of the general class of halocarbons, although the distinction is not often made. Haloalkanes are widely used commercially. They are used as flame retardants, fire extinguishants, refrigerants, propellants, solvents, and pharmaceuticals. Subsequent to the widespread use in commerce, many halocarbons have also been shown to be serious pollutants and toxins. For example, the chlorofluorocarbons have been shown to lead to ozone depletion. Methyl bromide is a controversial fumigant. Only haloalkanes that contain chlorine, bromine, and iodine are a threat to the ozone layer, but fluorinated volatile haloalkanes in theory may have activity as greenhouse gases. Methyl iodide, a naturally occurring substance, however, does not have ozone-depleting properties and the United States Environmental Protection Agency has designated the compound a non-ozone layer depleter. For more information, see Halomethane. Haloalkane or alkyl halides are the compounds which have the general formula "RX" where R is an alkyl or substituted alkyl group and X is a halogen.

In chemistry, the term phosphonium describes polyatomic cations with the chemical formula PR+
4. These cations have tetrahedral structures. The salts are generally colorless or take the color of the anions.

Triphenylphosphine (IUPAC name: triphenylphosphane) is a common organophosphorus compound with the formula P(C6H5)3 and often abbreviated to PPh3 or Ph3P. It is widely used in the synthesis of organic and organometallic compounds. PPh3 exists as relatively air stable, colorless crystals at room temperature. It dissolves in non-polar organic solvents such as benzene and diethyl ether.

Phosphorus triiodide (PI3) is an inorganic compound with the formula PI3. A red solid, it is too unstable to be stored; it is, nevertheless, commercially available. It is widely used in organic chemistry for converting alcohols to alkyl iodides. It is also a powerful reducing agent. Note that phosphorus also forms a lower iodide, P2I4, but the existence of PI5 is doubtful at room temperature.
In chemistry, there are three series of binary phosphorus halides, containing phosphorus in the oxidation states +5, +3 and +2. All compounds have been described, in varying degrees of detail, although serious doubts have been cast on the existence of PI5. Mixed chalcogen halides also exist.

Copper(I) iodide is the inorganic compound with the formula CuI. It is also known as cuprous iodide. It is useful in a variety of applications ranging from organic synthesis to cloud seeding.
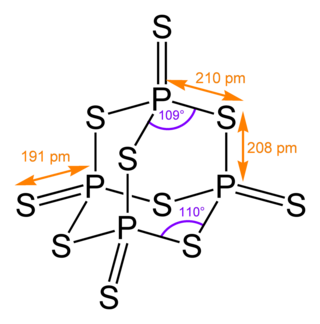
Phosphorus pentasulfide is the inorganic compound with the formula P2S5 (empirical) or P4S10 (molecular). This yellow solid is the one of two phosphorus sulfides of commercial value. Samples often appear greenish-gray due to impurities. It is soluble in carbon disulfide but reacts with many other solvents such as alcohols, DMSO, and DMF.

Aluminium iodide is a chemical compound containing aluminium and iodine. Invariably, the name refers to a compound of the composition AlI
3, formed by the reaction of aluminium and iodine or the action of HI on Al metal. The hexahydrate is obtained from a reaction between metallic aluminum or aluminum hydroxide with hydrogen iodide or hydroiodic acid. Like the related chloride and bromide, AlI
3 is a strong Lewis acid and will absorb water from the atmosphere. It is employed as a reagent for the scission of certain kinds of C-O and N-O bonds. It cleaves aryl ethers and deoxygenates epoxides.
Tin(II) bromide is a chemical compound of tin and bromine with a chemical formula of SnBr2. Tin is in the +2 oxidation state. The stability of tin compounds in this oxidation state is attributed to the inert pair effect.

Selenium compounds commonly exist in the oxidation states −2, +2, +4, and +6.
Iodine can form compounds using multiple oxidation states. Iodine is quite reactive, but it is much less reactive than the other halogens. For example, while chlorine gas will halogenate carbon monoxide, nitric oxide, and sulfur dioxide, iodine will not do so. Furthermore, iodination of metals tends to result in lower oxidation states than chlorination or bromination; for example, rhenium metal reacts with chlorine to form rhenium hexachloride, but with bromine it forms only rhenium pentabromide and iodine can achieve only rhenium tetraiodide. By the same token, however, since iodine has the lowest ionisation energy among the halogens and is the most easily oxidised of them, it has a more significant cationic chemistry and its higher oxidation states are rather more stable than those of bromine and chlorine, for example in iodine heptafluoride.
In inorganic chemistry, sulfonyl halide groups occur when a sulfonyl functional group is singly bonded to a halogen atom. They have the general formula RSO2X, where X is a halogen. The stability of sulfonyl halides decreases in the order fluorides > chlorides > bromides > iodides, all four types being well known. The sulfonyl chlorides and fluorides are of dominant importance in this series.
There are three sets of Indium halides, the trihalides, the monohalides, and several intermediate halides. In the monohalides the oxidation state of indium is +1 and their proper names are indium(I) fluoride, indium(I) chloride, indium(I) bromide and indium(I) iodide.

Thiophosphoryl chloride is an inorganic compound with the chemical formula PSCl3. It is a colorless pungent smelling liquid that fumes in air. It is synthesized from phosphorus chloride and used to thiophosphorylate organic compounds, such as to produce insecticides.

Diphosphorus tetraiodide is an orange crystalline solid with the formula P2I4. It has been used as a reducing agent in organic chemistry. It is a rare example of a compound with phosphorus in the +2 oxidation state, and can be classified as a subhalide of phosphorus. It is the most stable of the diphosphorus tetrahalides.

Thiophosphoryl fluoride is an inorganic molecular gas with formula PSF3 containing phosphorus, sulfur and fluorine. It spontaneously ignites in air and burns with a cool flame. The discoverers were able to have flames around their hands without discomfort, and called it "probably one of the coldest flames known". The gas was discovered in 1888.

Sulfinyl halide have the general formula R−S(O)−X, where X is a halogen. They are intermediate in oxidation level between sulfenyl halides, R−S−X, and sulfonyl halides, R−SO2−X. The best known examples are sulfinyl chlorides, thermolabile, moisture-sensitive compounds, which are useful intermediates for preparation of other sufinyl derivatives such as sulfinamides, sulfinates, sulfoxides, and thiosulfinates. Unlike the sulfur atom in sulfonyl halides and sulfenyl halides, the sulfur atom in sulfinyl halides is chiral, as shown for methanesulfinyl chloride.

Thiophosphoryl iodide is an inorganic compound with the formula PSI3.
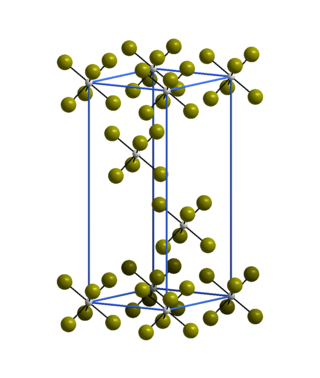
Tungsten hexabromide, also known as tungsten(VI) bromide, is a chemical compound of tungsten and bromine with the formula WBr6. It is an air-sensitive dark grey powder that decomposes above 200 °C to tungsten(V) bromide and bromine.
















