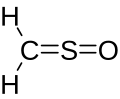 | |
| Names | |
|---|---|
| Preferred IUPAC name Methylidene-λ4-sulfanone | |
| Other names sulfine | |
| Identifiers | |
3D model (JSmol) | |
| ChemSpider | |
PubChem CID | |
CompTox Dashboard (EPA) | |
| |
| |
| Properties | |
| CH2OS | |
| Molar mass | 62.09 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Sulfinylmethane or sulfine is an organic compound with molecular formula H2CSO. It is the simplest sulfine. Sulfines are chemical compounds with the general structure XY=SO. [1] IUPAC considers the term 'sulfine' obsolete, [2] preferring instead thiocarbonyl S-oxide; despite this, the use of the term sulfine still predominates in the chemical literature.
