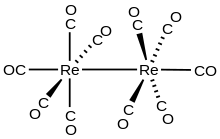
Rhenium is a chemical element; it has symbol Re and atomic number 75. It is a silvery-gray, heavy, third-row transition metal in group 7 of the periodic table. With an estimated average concentration of 1 part per billion (ppb), rhenium is one of the rarest elements in the Earth's crust. It has the third-highest melting point and second-highest boiling point of any element at 5869 K. It resembles manganese and technetium chemically and is mainly obtained as a by-product of the extraction and refinement of molybdenum and copper ores. It shows in its compounds a wide variety of oxidation states ranging from −1 to +7.

Group 7, numbered by IUPAC nomenclature, is a group of elements in the periodic table. It contains manganese (Mn), technetium (Tc), rhenium (Re) and bohrium (Bh). This group lies in the d-block of the periodic table, and are hence transition metals. This group is sometimes called the manganese group or manganese family after its lightest member; however, the group itself has not acquired a trivial name because it belongs to the broader grouping of the transition metals.

Perrhenic acid is the chemical compound with the formula Re2O7(H2O)2. It is obtained by evaporating aqueous solutions of Re2O7. Conventionally, perrhenic acid is considered to have the formula HReO4, and a species of this formula forms when rhenium(VII) oxide sublimes in the presence of water or steam. When a solution of Re2O7 is kept for a period of months, it breaks down and crystals of HReO4·H2O are formed, which contain tetrahedral ReO−4. For most purposes, perrhenic acid and rhenium(VII) oxide are used interchangeably. Rhenium can be dissolved in nitric or concentrated sulfuric acid to produce perrhenic acid.
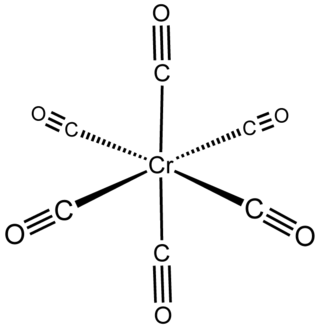
Chromium hexacarbonyl is a chromium(0) organometallic compound with the formula Cr(CO)6. It is a homoleptic complex, which means that all the ligands are identical. It is a colorless crystalline air-stable solid, with a high vapor pressure.

Metal carbonyls are coordination complexes of transition metals with carbon monoxide ligands. Metal carbonyls are useful in organic synthesis and as catalysts or catalyst precursors in homogeneous catalysis, such as hydroformylation and Reppe chemistry. In the Mond process, nickel tetracarbonyl is used to produce pure nickel. In organometallic chemistry, metal carbonyls serve as precursors for the preparation of other organometallic complexes.

Rhenium(VII) oxide is the inorganic compound with the formula Re2O7. This yellowish solid is the anhydride of HOReO3. Perrhenic acid, Re2O7·2H2O, is closely related to Re2O7. Re2O7 is the raw material for all rhenium compounds, being the volatile fraction obtained upon roasting the host ore.

Triiron dodecarbonyl is the organoiron compound with the formula Fe3(CO)12. It is a dark green solid that sublimes under vacuum. It is soluble in nonpolar organic solvents to give intensely green solutions. Most low-nuclearity clusters are pale yellow or orange. Hot solutions of Fe3(CO)12 decompose to an iron mirror, which can be pyrophoric in air.The solid decomposes slowly in air, and thus samples are typically stored cold under an inert atmosphere. It is a more reactive source of iron(0) than iron pentacarbonyl.

Dimanganese decacarbonyl, which has the chemical formula Mn2(CO)10, is a binary bimetallic carbonyl complex centered around the first row transition metal manganese. The first reported synthesis of Mn2(CO)10 was in 1954 at Linde Air Products Company and was performed by Brimm, Lynch, and Sesny. Their hypothesis about, and synthesis of, dimanganese decacarbonyl was fundamentally guided by the previously known dirhenium decacarbonyl (Re2(CO)10), the heavy atom analogue of Mn2(CO)10. Since its first synthesis, Mn2(CO)10 has been use sparingly as a reagent in the synthesis of other chemical species, but has found the most use as a simple system on which to study fundamental chemical and physical phenomena, most notably, the metal-metal bond. Dimanganese decacarbonyl is also used as a classic example to reinforce fundamental topics in organometallic chemistry like d-electron count, the 18-electron rule, oxidation state, valency, and the isolobal analogy.
Walter Hieber was an inorganic chemist, known as the father of metal carbonyl chemistry. He was born 18 December 1895 and died 29 November 1976. Hieber's father was Johannes Hieber, an influential evangelical minister and politician.

Bromopentacarbonylrhenium(I) is an inorganic compound of rhenium, commonly used for the syntheses of other rhenium complexes.
Organorhenium chemistry describes the compounds with Re−C bonds. Because rhenium is a rare element, relatively few applications exist, but the area has been a rich source of concepts and a few useful catalysts.

Cobalt tetracarbonyl hydride is an organometallic compound with the formula HCo(CO)4. It is a volatile, yellow liquid that forms a colorless vapor and has an intolerable odor. The compound readily decomposes upon melt and in absentia of high CO partial pressures forms Co2(CO)8. Despite operational challenges associated with its handling, the compound has received considerable attention for its ability to function as a catalyst in hydroformylation. In this respect, HCo(CO)4 and related derivatives have received significant academic interest for their ability to mediate a variety of carbonylation (introduction of CO into inorganic compounds) reactions.
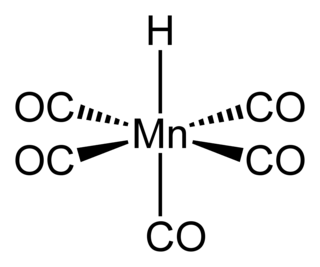
Pentacarbonylhydridomanganese is an organometallic compound with formula HMn(CO)5. This compound is one of the most stable "first-row" transition metal hydrides.

Iron tetracarbonyl dihydride is the organometallic compound with the formula H2Fe(CO)4. This compound was the first transition metal hydride discovered. The complex is stable at low temperatures but decomposes rapidly at temperatures above –20 °C.

The Tolman electronic parameter (TEP) is a measure of the electron donating or withdrawing ability of a ligand. It is determined by measuring the frequency of the A1 C-O vibrational mode (ν(CO)) of a (pseudo)-C3v symmetric complex, [LNi(CO)3] by infrared spectroscopy, where L is the ligand of interest. [LNi(CO)3] was chosen as the model compound because such complexes are readily prepared from tetracarbonylnickel(0). The shift in ν(CO) is used to infer the electronic properties of a ligand, which can aid in understanding its behavior in other complexes. The analysis was introduced by Chadwick A. Tolman.

Cyclopentadienyliron dicarbonyl dimer is an organometallic compound with the formula [(η5-C5H5)Fe(CO)2]2, often abbreviated to Cp2Fe2(CO)4, [CpFe(CO)2]2 or even Fp2, with the colloquial name "fip dimer". It is a dark reddish-purple crystalline solid, which is readily soluble in moderately polar organic solvents such as chloroform and pyridine, but less soluble in carbon tetrachloride and carbon disulfide. Cp2Fe2(CO)4 is insoluble in but stable toward water. Cp2Fe2(CO)4 is reasonably stable to storage under air and serves as a convenient starting material for accessing other Fp (CpFe(CO)2) derivatives (described below).

Metal carbonyl hydrides are complexes of transition metals with carbon monoxide and hydride as ligands. These complexes are useful in organic synthesis as catalysts in homogeneous catalysis, such as hydroformylation.

Transition metal nitrile complexes are coordination compounds containing nitrile ligands. Because nitriles are weakly basic, the nitrile ligands in these complexes are often labile.

Transition metal acyl complexes describes organometallic complexes containing one or more acyl (RCO) ligands. Such compounds occur as transient intermediates in many industrially useful reactions, especially carbonylations.
Rhenium compounds are compounds formed by the transition metal rhenium (Re). Rhenium can form in many oxidation states, and compounds are known for every oxidation state from -3 to +7 except -2, although the oxidation states +7, +4, and +3 are the most common. Rhenium is most available commercially as salts of perrhenate, including sodium and ammonium perrhenates. These are white, water-soluble compounds. The tetrathioperrhenate anion [ReS4]− is possible.