
Group 7, numbered by IUPAC nomenclature, is a group of elements in the periodic table. It contains manganese (Mn), technetium (Tc), rhenium (Re) and bohrium (Bh). This group lies in the d-block of the periodic table, and are hence transition metals. This group is sometimes called the manganese group or manganese family after its lightest member; however, the group itself has not acquired a trivial name because it belongs to the broader grouping of the transition metals.

Selenium tetrafluoride (SeF4) is an inorganic compound. It is a colourless liquid that reacts readily with water. It can be used as a fluorinating reagent in organic syntheses (fluorination of alcohols, carboxylic acids or carbonyl compounds) and has advantages over sulfur tetrafluoride in that milder conditions can be employed and it is a liquid rather than a gas.

Tungsten oxytetrafluoride is an inorganic compound with the formula WOF4. It is a colorless diamagnetic solid. The compound is one of many oxides of tungsten. It is usually encountered as product of the partial hydrolysis of tungsten hexafluoride.

Technetium hexafluoride or technetium(VI) fluoride (TcF6) is a yellow inorganic compound with a low melting point. It was first identified in 1961. In this compound, technetium has an oxidation state of +6, the highest oxidation state found in the technetium halides. In this respect, technetium differs from rhenium, which forms a heptafluoride, ReF7. Technetium hexafluoride occurs as an impurity in uranium hexafluoride, as technetium is a fission product of uranium (spontaneous fission in natural uranium, possible contamination from induced fission inside the reactor in reprocessed uranium). The fact that the boiling point of the hexafluorides of uranium and technetium are very close to each other presents a problem in using fluoride volatility in nuclear reprocessing.
Technetium compounds are chemical compounds containing the chemical element technetium. Technetium can form multiple oxidation states, but often forms in the +4 and +7 oxidation states. Because technetium is radioactive, technetium compounds are extremely rare on Earth.
Rhenium trioxide or rhenium(VI) oxide is an inorganic compound with the formula ReO3. It is a red solid with a metallic lustre that resembles copper in appearance. It is the only stable trioxide of the Group 7 elements (Mn, Tc, Re).
A hexafluoride is a chemical compound with the general formula QXnF6, QXnF6m−, or QXnF6m+. Many molecules fit this formula. An important hexafluoride is hexafluorosilicic acid (H2SiF6), which is a byproduct of the mining of phosphate rock. In the nuclear industry, uranium hexafluoride (UF6) is an important intermediate in the purification of this element.

Chromyl fluoride is an inorganic compound with the formula CrO2F2. It is a violet-red colored crystalline solid that melts to an orange-red liquid.

Rhenium hexafluoride, also rhenium(VI) fluoride, (ReF6) is a compound of rhenium and fluorine and one of the seventeen known binary hexafluorides.

Bromine monofluoride is a quite unstable interhalogen compound with the chemical formula BrF. It can be produced through the reaction of bromine trifluoride (or bromine pentafluoride) and bromine. Due to its lability, the compound can be detected but not isolated:

Osmium hexafluoride, also osmium(VI) fluoride, (OsF6) is a compound of osmium and fluorine, and one of the seventeen known binary hexafluorides.
Fluorine forms a great variety of chemical compounds, within which it always adopts an oxidation state of −1. With other atoms, fluorine forms either polar covalent bonds or ionic bonds. Most frequently, covalent bonds involving fluorine atoms are single bonds, although at least two examples of a higher order bond exist. Fluoride may act as a bridging ligand between two metals in some complex molecules. Molecules containing fluorine may also exhibit hydrogen bonding. Fluorine's chemistry includes inorganic compounds formed with hydrogen, metals, nonmetals, and even noble gases; as well as a diverse set of organic compounds. For many elements the highest known oxidation state can be achieved in a fluoride. For some elements this is achieved exclusively in a fluoride, for others exclusively in an oxide; and for still others the highest oxidation states of oxides and fluorides are always equal.

Chlorine oxide trifluoride or chlorine trifluoride oxide is a corrosive liquid molecular compound with formula ClOF3. It was developed secretly as a rocket fuel oxidiser.
Nitride fluorides containing nitride and fluoride ions with the formula NF4-. They can be electronically equivalent to a pair of oxide ions O24-. Nitride fluorides were discovered in 1996 by Lavalle et al. They heated diammonium technetium hexafluoride to 300 °C to yield TcNF. Another preparation is to heat a fluoride compound with a nitride compound in a solid state reaction. The fluorimido ion is F-N2- and is found in a rhenium compound.
Rhenium compounds are compounds formed by the transition metal rhenium (Re). Rhenium can form in many oxidation states, and compounds are known for every oxidation state from -3 to +7 except -2, although the oxidation states +7, +4, and +3 are the most common. Rhenium is most available commercially as salts of perrhenate, including sodium and ammonium perrhenates. These are white, water-soluble compounds. The tetrathioperrhenate anion [ReS4]− is possible.
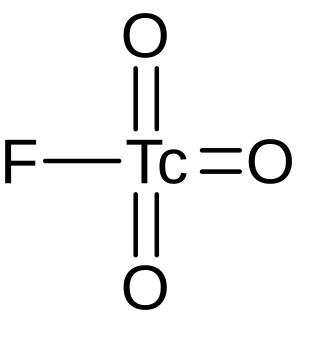
Pertechnetyl fluoride is an inorganic compound, a salt of technetium and hydrofluoric acid with the chemical formula TcO
3F. The compound was originally synthesized by H. Selig and G. Malm in 1963.
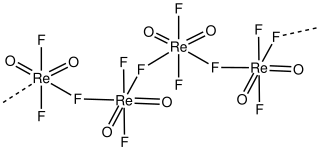
Rhenium dioxide trfluoride is an inorganic compound with the formula ReO2F3. A white diamagnetic solid, it one of the few oxyfluorides of rhenium, another being rhenium trioxide fluoride, ReO3F. The material is of some academic interest as a rare example of an dioxide trifluoride. It can be prepared by the reaction of xenon difluoride and rhenium trioxide chloride:
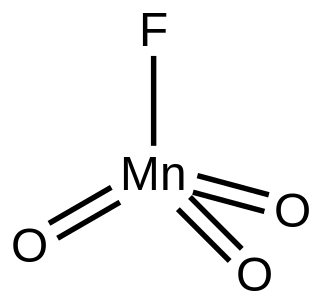
Manganese trioxide fluoride is an inorganic compound with the formula MnO3F. A green diamagnetic liquid, the compound has no applications, but it is of some academic interest as a rare example of a metal trioxide fluoride.

Rhenium trioxide chloride is an inorganic compound with the formula ReO3Cl. It is a colorless, distillable, diamagnetic liquid. It is a rhenium oxychloride. The material is used as a reagent in the preparation of rhenium compounds.
Rhenium oxyfluoride may refer to:













