
Dysprosium(III) chloride (DyCl3), also known as dysprosium trichloride, is a compound of dysprosium and chlorine. It is a white to yellow solid which rapidly absorbs water on exposure to moist air to form a hexahydrate, DyCl3·6H2O. Simple rapid heating of the hydrate causes partial hydrolysis to an oxychloride, DyOCl.

Iron(II) chloride, also known as ferrous chloride, is the chemical compound of formula FeCl2. It is a paramagnetic solid with a high melting point. The compound is white, but typical samples are often off-white. FeCl2 crystallizes from water as the greenish tetrahydrate, which is the form that is most commonly encountered in commerce and the laboratory. There is also a dihydrate. The compound is highly soluble in water, giving pale green solutions.

Palladium(II) chloride, also known as palladium dichloride and palladous chloride, are the chemical compounds with the formula PdCl2. PdCl2 is a common starting material in palladium chemistry – palladium-based catalysts are of particular value in organic synthesis. It is prepared by the reaction of chlorine with palladium metal at high temperatures.
Octahedral clusters are inorganic or organometallic cluster compounds composed of six metals in an octahedral array. Many types of compounds are known, but all are synthetic.

Tantalum(V) chloride, also known as tantalum pentachloride, is an inorganic compound with the formula TaCl5. It takes the form of a white powder and is commonly used as a starting material in tantalum chemistry. It readily hydrolyzes to form tantalum(V) oxychloride (TaOCl3) and eventually tantalum pentoxide (Ta2O5); this requires that it be synthesised and manipulated under anhydrous conditions, using air-free techniques.

Molybdenum(V) chloride is the inorganic compound with the empirical formula MoCl5. This dark volatile solid is used in research to prepare other molybdenum compounds. It is moisture-sensitive and soluble in chlorinated solvents.

Tungsten hexachloride is the chemical compound of tungsten and chlorine with the formula WCl6. This dark violet blue species exists as a volatile solid under standard conditions. It is an important starting reagent in the preparation of tungsten compounds. Other examples of charge-neutral hexachlorides are rhenium(VI) chloride and molybdenum(VI) chloride. The highly volatile tungsten hexafluoride is also known.

Tungsten dichloride dioxide, or Tungstyl chloride is the chemical compound with the formula WO2Cl2. It is a yellow-colored solid. It is used as a precursor to other tungsten compounds. Like other tungsten halides, WO2Cl2 is sensitive to moisture, undergoing hydrolysis.

Molybdenum dichloride describes chemical compounds with the empirical formula MoCl2. At least two forms are known, and both have attracted much attention from academic researchers because of the unexpected structures seen for these compounds and the fact that they give rise to hundreds of derivatives. The form discussed here is Mo6Cl12. The other molybdenum(II) chloride is potassium octachlorodimolybdate.

Molybdenum tetrachloride is the inorganic compound with the empirical formula MoCl4. The material exists as two polymorphs, both being dark-colored paramagnetic solids. These compounds are mainly of interest as precursors to other molybdenum complexes.

Molybdenum(III) chloride is the inorganic compound with the formula MoCl3. It forms purple crystals.
Tungsten(IV) chloride is an inorganic compound with the formula WCl4. It is a diamagnetic black solid. The compound is of interest in research as one of a handful of binary tungsten chlorides.

Transition metal nitrile complexes are coordination compounds containing nitrile ligands. Because nitriles are weakly basic, the nitrile ligands in these complexes are often labile.

Tungsten(III) chloride is the inorganic compound with the formula W6Cl18. It is a cluster compound. It is a brown solid, obtainable by chlorination of tungsten(II) chloride. Featuring twelve doubly bridging chloride ligands, the cluster adopts a structure related to the corresponding chlorides of niobium and tantalum. In contrast, W6Cl12 features eight triply bridging chlorides.

Tantalum(III) chloride or tantalum trichloride is non-stoichiometric with a range of composition from TaCl2.9 to TaCl3.1 Anionic and neutral clusters containing Ta(III) chloride include [Ta6Cl18]4− and [Ta6Cl14](H2O)4.
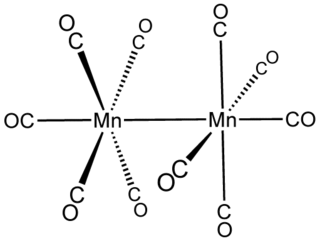
In inorganic chemistry, metal–metal bonds describe attractive interactions between metal centers. The simplest examples are found in bimetallic complexes. Metal–metal bonds can be "supported", i.e. be accompanied by one or more bridging ligands, or "unsupported". They can also vary according to bond order. The topic of metal–metal bonding is usually discussed within the framework of coordination chemistry, but the topic is related to extended metallic bonding, which describes interactions between metals in extended solids such as bulk metals and metal subhalides.

Hexa(tert-butoxy)ditungsten(III) is a coordination complex of tungsten(III). It is one of the homoleptic alkoxides of tungsten. A red, air-sensitive solid, the complex has attracted academic attention as the precursor to many organotungsten derivatives. It an example of a charge-neutral complex featuring a W≡W bond, arising from the coupling of a pair of d3 metal centers. It has attracted particular attention for its reactions with alkynes, leading to alkyne metathesis.

In chemistry, a transition metal chloride complex is a coordination complex that consists of a transition metal coordinated to one or more chloride ligand. The class of complexes is extensive.
Carbide chlorides are mixed anion compounds containing chloride anions and anions consisting entirely of carbon. In these compounds there is no bond between chlorine and carbon. But there is a bond between a metal and carbon. Many of these compounds are cluster compounds, in which metal atoms encase a carbon core, with chlorine atoms surrounding the cluster. The chlorine may be shared between clusters to form polymers or layers. Most carbon chloride compounds contain rare earth elements. Some are known from group 4 elements. The hexatungsten carbon cluster can be oxidised and reduced, and so have different numbers of chlorine atoms included.

In chemistry, a transition metal ether complex is a coordination complex consisting of a transition metal bonded to one or more ether ligand. The inventory of complexes is extensive. Common ether ligands are diethyl ether and tetrahydrofuran. Common chelating ether ligands include the glymes, dimethoxyethane (dme) and diglyme, and the crown ethers. Being lipophilic, metal-ether complexes often exhibit solubility in organic solvents, a property of interest in synthetic chemistry. In contrast, the di-ether 1,4-dioxane is generally a bridging ligand.
![Structure of the cluster anion [W6Cl14] Mo6Cl14-anion-from-xtal-2008-CM-3D-ellipsoids.png](//upload.wikimedia.org/wikipedia/commons/thumb/6/69/Mo6Cl14-anion-from-xtal-2008-CM-3D-ellipsoids.png/180px-Mo6Cl14-anion-from-xtal-2008-CM-3D-ellipsoids.png)
![Structure of the cluster anion [W6Cl14] Mo6Cl14-anion-from-xtal-2008-CM-3D-ellipsoids.png](http://upload.wikimedia.org/wikipedia/commons/thumb/6/69/Mo6Cl14-anion-from-xtal-2008-CM-3D-ellipsoids.png/180px-Mo6Cl14-anion-from-xtal-2008-CM-3D-ellipsoids.png)
















