α-Tungsten hexachloride | |||
 β-Tungsten hexachloride | |||
| |||
| Names | |||
|---|---|---|---|
| IUPAC names Tungsten hexachloride Tungsten(VI) chloride | |||
| Identifiers | |||
3D model (JSmol) | |||
| ChemSpider | |||
| ECHA InfoCard | 100.032.980 | ||
| EC Number |
| ||
PubChem CID | |||
| RTECS number |
| ||
| UNII | |||
CompTox Dashboard (EPA) | |||
| |||
| |||
| Properties | |||
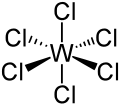
| WCl6 | |||
| Molar mass | 396.54 g·mol−1 | ||
| Appearance | dark blue crystals, moisture sensitive | ||
| Density | 3.52 g/cm3 | ||
| Melting point | 275 °C (527 °F; 548 K) | ||
| Boiling point | 346.7 °C (656.1 °F; 619.8 K) | ||
| Hydrolyzes | |||
| Solubility in chlorocarbons | soluble | ||
| −71.0·10−6 cm3/mol | |||
| Structure | |||
| α:rhombohedral, β: hexagonal | |||
| Octahedral | |||
| 0 D | |||
| Hazards | |||
| Occupational safety and health (OHS/OSH): | |||
Main hazards | oxidizer; hydrolysis releases HCl | ||
| Related compounds | |||
Other anions | |||
Other cations | |||
Related compounds | |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
Tungsten hexachloride is an inorganic chemical compound of tungsten and chlorine with the chemical formula W Cl 6. This dark violet-blue compound exists as volatile crystals under standard conditions. It is an important starting reagent in the preparation of tungsten compounds. [1] Other examples of charge-neutral hexachlorides are rhenium(VI) chloride and molybdenum(VI) chloride. The highly volatile tungsten hexafluoride is also known.
Contents
As a d0 atom, tungsten hexachloride is diamagnetic.