Triphenylphosphine (IUPAC name: triphenylphosphane) is a common organophosphorus compound with the formula P(C6H5)3 and often abbreviated to PPh3 or Ph3P. It is versatile compound that is widely used as a reagent in organic synthesis and as a ligand for transition metal complexes, including ones that serve as catalysts in organometallic chemistry. PPh3 exists as relatively air stable, colorless crystals at room temperature. It dissolves in non-polar organic solvents such as benzene and diethyl ether.

18-Crown-6 is an organic compound with the formula [C2H4O]6 and the IUPAC name of 1,4,7,10,13,16-hexaoxacyclooctadecane. It is a white, hygroscopic crystalline solid with a low melting point. Like other crown ethers, 18-crown-6 functions as a ligand for some metal cations with a particular affinity for potassium cations (binding constant in methanol: 106 M−1). The point group of 18-crown-6 is S6. The dipole moment of 18-crown-6 is solvent- and temperature-dependent. Below 25 °C, the dipole moment of 18-crown-6 is 2.76 ± 0.06 D in cyclohexane and 2.73 ± 0.02 in benzene. The synthesis of the crown ethers led to the awarding of the Nobel Prize in Chemistry to Charles J. Pedersen.

Dithiolene metal complexes are complexes containing 1,2-dithiolene ligands. 1,2-Dithiolene ligands, a particular case of 1,2-dichalcogenolene species along with 1,2-diselenolene derivatives, are unsaturated bidentate ligand wherein the two donor atoms are sulfur. 1,2-Dithiolene metal complexes are often referred to as "metal dithiolenes", "metallodithiolenes" or "dithiolene complexes". Most molybdenum- and tungsten-containing proteins have dithiolene-like moieties at their active sites, which feature the so-called molybdopterin cofactor bound to the Mo or W.

1,2-Bis(diphenylphosphino)ethane (dppe) is an organophosphorus compound with the formula (Ph2PCH2)2 (Ph = phenyl). It is a common symmetrical bidentate ligand in coordination chemistry. It is a white solid that is soluble in organic solvents.

Titanium(II) chloride is the chemical compound with the formula TiCl2. The black solid has been studied only moderately, probably because of its high reactivity. Ti(II) is a strong reducing agent: it has a high affinity for oxygen and reacts irreversibly with water to produce H2. The usual preparation is the thermal disproportionation of TiCl3 at 500 °C. The reaction is driven by the loss of volatile TiCl4:
A diamine is an amine with exactly two amino groups. Diamines are used as monomers to prepare polyamides, polyimides, and polyureas. The term diamine refers mostly to primary diamines, as those are the most reactive.

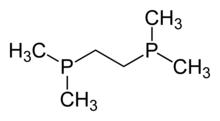
Diphosphines, sometimes called bisphosphanes, are organophosphorus compounds most commonly used as bidentate phosphine ligands in inorganic and organometallic chemistry. They are identified by the presence of two phosphino groups linked by a backbone, and are usually chelating. A wide variety of diphosphines have been synthesized with different linkers and R-groups. Alteration of the linker and R-groups alters the electronic and steric properties of the ligands which can result in different coordination geometries and catalytic behavior in homogeneous catalysts.

Diphenylphosphine, also known as diphenylphosphane, is an organophosphorus compound with the formula (C6H5)2PH. This foul-smelling, colorless liquid is easily oxidized in air. It is a precursor to organophosphorus ligands for use as catalysts.

Acetylenediol, or ethynediol, is a chemical substance with formula HO−C≡C−OH (an ynol). It is the diol of acetylene. Acetylenediol is unstable in the condensed phase, although its tautomer glyoxal (CHO)2 is well known.

Oxazoline is a five-membered heterocyclic organic compound with the formula C3H5NO. It is the parent of a family of compounds called oxazolines, which contain non-hydrogenic substituents on carbon and/or nitrogen. Oxazolines are the unsaturated analogues of oxazolidines, and they are isomeric with isoxazolines, where the N and O are directly bonded. Two isomers of oxazoline are known, depending on the location of the double bond.

Chadwick Alma Tolman was an American chemist. He obtained his B.S. in Chemistry from Massachusetts Institute of Technology. He earned his Ph.D. in Chemistry as a microwave spectroscopist from U.C. Berkeley under the guidance of William Dulaney Gwinn.

A metal-phosphine complex is a coordination complex containing one or more phosphine ligands. Almost always, the phosphine is an organophosphine of the type R3P (R = alkyl, aryl). Metal phosphine complexes are useful in homogeneous catalysis. Prominent examples of metal phosphine complexes include Wilkinson's catalyst (Rh(PPh3)3Cl), Grubbs' catalyst, and tetrakis(triphenylphosphine)palladium(0).

trans-Bis(dinitrogen)bis[1,2-bis(diphenylphosphino)ethane]molybdenum(0) is a coordination complex with the formula Mo(N2)2(dppe)2. It is a relatively air stable yellow-orange solid. It is notable as being the first discovered dinitrogen containing complex of molybdenum.

Chlorobis(dppe)iron hydride is a coordination complex with the formula HFeCl(dppe)2, where dppe is the bidentate ligand 1,2-bis(diphenylphosphino)ethane. It is a red-violet solid. The compound has attracted much attention as a precursor to dihydrogen complexes.
Gregory S. Girolami is the William H. and Janet G. Lycan Professor of Chemistry at the University of Illinois Urbana-Champaign. His research focuses on the synthesis, properties, and reactivity of new inorganic, organometallic, and solid state species. Girolami has been elected a fellow of the American Association for the Advancement of Science, the Royal Society of Chemistry, and the American Chemical Society.

Bis(dicyclohexylphosphino)ethane, abbreviated dcpe, is an organophosphorus compound with the formula (C6H11)2PCH2CH2P(C6H11)2. It is a white solid that is soluble in nonpolar organic solvents. The compound is used as a bulky and highly basic diphosphine ligand in coordination chemistry.
In organophosphorus chemistry, aminophosphines are compounds with the formula R3−nP(NR2)n where R is a hydrogen or organic substituent, and n = 0, 1, or 2. At one extreme, the parents H2PNH2 and P(NH2)3 are lightly studied and fragile. At the other extreme, tris(dimethylamino)phosphine (P(NMe2)3) is commonly available. Intermediate members are known, such as Ph2PN(H)Ph. Aminophosphines are typically colorless and reactive to oxygen. Aminophosphines are pyramidal geometry at phosphorus.
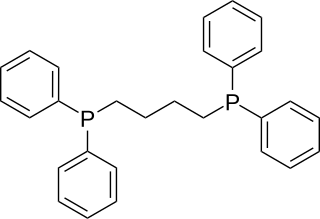
1,4-Bis(diphenylphosphino)butane (dppb) is an organophosphorus compound with the formula (Ph2PCH2CH2)2. It is less commonly used in coordination chemistry than other diphosphine ligands such as dppe. It is a white solid that is soluble in organic solvents.
1,2-Bis(dichlorophosphino)ethane is an organophosphorus compound with the formula (CH2PCl2)2. This colorless liquid is a precursor to chelating diphosphines.

1,1,1-Tris(diphenylphosphinomethyl)ethane, also called Triphos, is an organophosphorus compound with the formula CH3C[CH2PPh2]3. An air-sensitive white solid, it is a tripodal ligand ("three-legged") of idealized C3v symmetry. It was originally prepared by the reaction of sodium diphenylphosphide and CH3C(CH2Cl)3: