
Acylureas (also called N-acylureas or ureides) are a class of chemical compounds formally derived from the acylation of urea. [1]

Acylureas (also called N-acylureas or ureides) are a class of chemical compounds formally derived from the acylation of urea. [1]
A subclass of acylureas known as benzoylureas are insecticides. They act as insect growth regulators by inhibiting the synthesis of chitin resulting in weakened cuticles and preventing molting. [2] Members of this class include diflubenzuron, flufenoxuron, hexaflumuron, lufenuron, and teflubenzuron.
The acylurea functional group is also found in some pharmaceutical drugs such as the anticonvulsants phenacemide, pheneturide, chlorphenacemide, and acetylpheneturide (which are phenylureides), [3] and the sedatives acecarbromal, bromisoval, and carbromal (which are bromoureides). Others include apronal (apronalide), capuride, and ectylurea. Barbiturates (a class of cyclic ureas) are structurally and mechanistically related to them. [4] The phenylureides are also closely related to the hydantoins, such as phenytoin, and may be considered ring-opened analogues of them. [5]
A diureide is a complex nitrogenous substance regarded as containing two molecules of urea or their radicals, e.g. uric acid or allantoin.
Hydantoin, or glycolylurea, can be considered the cyclic form of acylurea.

Phenylpropanolamine (PPA) is a sympathomimetic agent which is used as a decongestant and appetite suppressant. It was commonly used in prescription and over-the-counter cough and cold preparations. In veterinary medicine, it is used to control urinary incontinence in dogs.
Hydantoin, or glycolylurea, is a heterocyclic organic compound with the formula CH2C(O)NHC(O)NH. It is a colorless solid that arises from the reaction of glycolic acid and urea. It is an oxidized derivative of imidazolidine. In a more general sense, hydantoins can refer to a groups and a class of compounds with the same ring structure as the parent. For example, phenytoin (mentioned below) has two phenyl groups substituted onto the number 5 carbon in a hydantoin molecule.
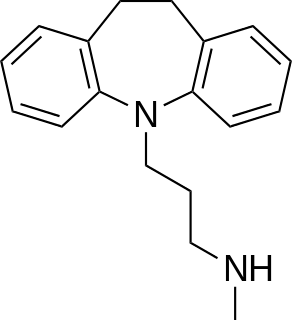
Desipramine, sold under the brand name Norpramin among others, is a tricyclic antidepressant (TCA) used in the treatment of depression. It acts as a relatively selective norepinephrine reuptake inhibitor, though it does also have other activities such as weak serotonin reuptake inhibitory, α1-blocking, antihistamine, and anticholinergic effects. The drug is not considered a first-line treatment for depression since the introduction of selective serotonin reuptake inhibitor (SSRI) antidepressants, which have fewer side effects and are safer in overdose.

Ketotifen, sold under the brand name Zaditor among others, is a second-generation noncompetitive H1-antihistamine and mast cell stabilizer. It is most commonly sold as a salt with fumaric acid, ketotifen fumarate, and is available in two forms. In its ophthalmic form, it is used to treat allergic conjunctivitis. In its oral form, it is used to prevent asthma attacks or anaphylaxis, as well as various mast cell, allergic-type disorders.

Aminosteroids are a group of steroids with a similar structure based on an amino-substituted steroid nucleus. They are neuromuscular blocking agents, acting as competitive antagonists of the nicotinic acetylcholine receptor (nAChR), and block the signaling of acetylcholine in the nervous system. These drugs include candocuronium iodide, dacuronium bromide, dihydrochandonium, dipyrandium, malouetine, pancuronium bromide, pipecuronium bromide, rapacuronium bromide, rocuronium bromide, stercuronium iodide, and vecuronium bromide.

Butriptyline, sold under the brand name Evadyne among others, is a tricyclic antidepressant (TCA) that has been used in the United Kingdom and several other European countries for the treatment of depression but appears to no longer be marketed. Along with trimipramine, iprindole, and amoxapine, it has been described as an "atypical" or "second-generation" TCA due to its relatively late introduction and atypical pharmacology. It was very little-used compared to other TCAs, with the number of prescriptions dispensed only in the thousands.
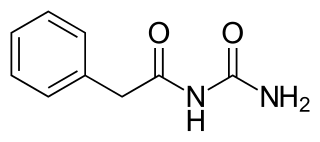
Phenacemide, also known as phenylacetylurea, is an anticonvulsant of the ureide (acetylurea) class. It is a congener and ring-opened analogue of phenytoin, and is structurally related to the barbiturates and to other hydantoins. Phenacemide was introduced in 1949 for the treatment of epilepsy, but was eventually withdrawn due to toxicity.

Fluoxymesterone, sold under the brand names Halotestin and Ultandren among others, is an androgen and anabolic steroid (AAS) medication which is used in the treatment of low testosterone levels in men, delayed puberty in boys, breast cancer in women, and anemia. It is taken by mouth.

Metitepine, also known as methiothepin, is a drug described as a "psychotropic agent" of the tricyclic group which was never marketed. It acts as a non-selective antagonist of serotonin, dopamine, and adrenergic receptors and has antipsychotic properties.
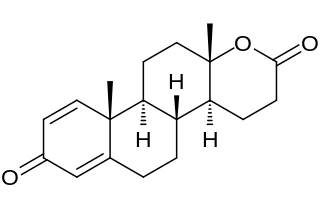
Testolactone is a non-selective, irreversible, steroidal aromatase inhibitor which is used as an antineoplastic drug to treat advanced-stage breast cancer. The drug was discontinued in 2008 and is no longer available for medical use. However, it has been reported to still be marketed in the United States by Bristol-Myers Squibb under the brand name Teslac.
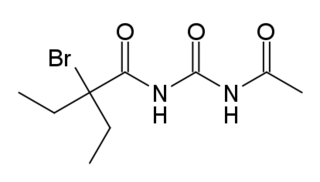
Acecarbromal (INN), also known as acetylcarbromal and acetyladalin, is a hypnotic and sedative drug of the ureide (acylurea) group discovered by Bayer in 1917 that was formerly marketed in the United States and Europe. It is also used in combination with extract of quebracho and vitamin E as a treatment for erectile dysfunction under the brand name Afrodor in Europe. Acecarbromal is structurally related to the barbiturates, which are basically cyclized ureas. Prolonged use is not recommended as it can cause bromine poisoning.

A norepinephrine releasing agent (NRA), also known as an adrenergic releasing agent, is a catecholaminergic type of drug which induces the release of norepinephrine (noradrenaline) and epinephrine (adrenaline) from the pre-synaptic neuron into the synapse. This in turn leads to increased extracellular concentrations of norepinephrine and epinephrine therefore an increase in adrenergic neurotransmission.
Substituted arylalkylamines are a group of chemical compounds. Two major classes of arylalkylamines include indolylalkylamines and phenylalkylamines, which consist of the monoamine neurotransmitters as well as clinically-used and recreationally-abused monoaminergic drugs, including psychostimulants, anorectics, wakefulness-promoting agents, bronchodilators, decongestants, antidepressants, entactogens, and psychedelics, among others.
α-Alkyltryptamines are a group of substituted tryptamines which possess an alkyl group, such as a methyl or ethyl group, attached at the alpha carbon, and in most cases no substitution on the amine nitrogen. α-Alkylation of tryptamine makes it much more metabolically stable and resistant to degradation by monoamine oxidase, resulting in increased potency and greatly lengthened half-life. This is analogous to α-methylation of phenethylamine into amphetamine.
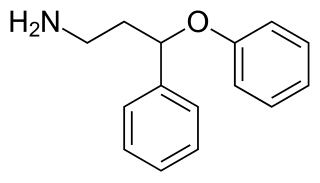
PPPA, or 3-phenoxy-3-phenylpropan-1-amine, is a drug which is described as an antidepressant. It was derived by Eli Lilly from the antihistamine diphenhydramine, a 2-diphenylmethoxyethanamine derivative with additional properties as a selective serotonin reuptake inhibitor (SSRI), and has been the basis for the subsequent discovery of a number of other antidepressant drugs.

Doisynolic acid is a synthetic, nonsteroidal, orally active estrogen that was never marketed. The reaction of estradiol or estrone with potassium hydroxide, a strong base, results in doisynolic acid as a degradation product, which retains high estrogenic activity, and this reaction was how the drug was discovered, in the late 1930s. The drug is a highly active and potent estrogen by the oral or subcutaneous route. The reaction of equilenin or dihydroequilenin with potassium hydroxide was also found to produce bisdehydrodoisynolic acid, the levorotatory isomer of which is an estrogen with an "astonishingly" high degree of potency, while the dextrorotatory isomer is inactive. Doisynolic acid was named after Edward Adelbert Doisy, a pioneer in the field of estrogen research and one of the discoverers of estrone.

A prolactin modulator is a drug which modulates the secretion of the pituitary hormone prolactin from the anterior pituitary gland. Prolactin inhibitors suppress and prolactin releasers induce the secretion of prolactin, respectively.

Norclomipramine, also known as N-desmethylclomipramine and chlordesipramine, is the major active metabolite of the tricyclic antidepressant (TCA) clomipramine (Anafranil).

Northiaden, also known as N-desmethyldosulepin, is the major active metabolite of the tricyclic antidepressant (TCA) dosulepin.
In pharmacology and pharmaceutics, a prototype drug is an individual drug that represents a drug class – group of medications having similar chemical structures, mechanism of action and mode of action. Prototypes are the most important, and typically the first developed drugs within the class, and are used as a reference to which all other drugs are compared.