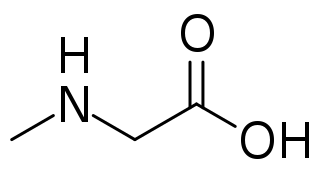
Sarcosine, also known as N-methylglycine, or monomethylglycine, is a amino acid with the formula CH3N(H)CH2CO2H. It exists at neutral pH as the zwitterion CH3N+(H)2CH2CO2−, which can be obtained as a white, water-soluble powder. Like some amino acids, sarcosine converts to a cation at low pH and an anion at high pH, with the respective formulas CH3N+(H)2CH2CO2H and CH3N(H)CH2CO2−. Sarcosine is a close relative of glycine, with a secondary amine in place of the primary amine.

The norepinephrine transporter (NET), also known as noradrenaline transporter (NAT), is a protein that in humans is encoded by the solute carrier family 6 member 2 (SLC6A2) gene.
Neurotransmitter transporters are a class of membrane transport proteins that span the cellular membranes of neurons. Their primary function is to carry neurotransmitters across these membranes and to direct their further transport to specific intracellular locations. There are more than twenty types of neurotransmitter transporters.
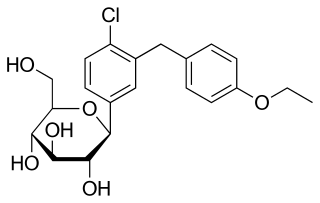
Dapagliflozin, sold under the brand names Farxiga (US) and Forxiga (EU) among others, is a medication used to treat type 2 diabetes. It is also used to treat adults with heart failure and chronic kidney disease. It reversibly inhibits sodium-glucose co-transporter 2 (SGLT-2) in the renal proximal convoluted tubule to reduce glucose reabsorption and increase urinary glucose excretion.

Sodium- and chloride-dependent glycine transporter 1, also known as glycine transporter 1, is a protein that in humans is encoded by the SLC6A9 gene which is promising therapeutic target for treatment of diabetes and obesity.

Reuptake inhibitors (RIs) are a type of reuptake modulators. It is a drug that inhibits the plasmalemmal transporter-mediated reuptake of a neurotransmitter from the synapse into the pre-synaptic neuron. This leads to an increase in extracellular concentrations of the neurotransmitter and an increase in neurotransmission. Various drugs exert their psychological and physiological effects through reuptake inhibition, including many antidepressants and psychostimulants.

Remogliflozin etabonate (INN/USAN) is a drug of the gliflozin class for the treatment of non-alcoholic steatohepatitis ("NASH") and type 2 diabetes. Remogliflozin was discovered by the Japanese company Kissei Pharmaceutical and is currently being developed by BHV Pharma, a wholly owned subsidiary of North Carolina, US-based Avolynt, and Glenmark Pharmaceuticals through a collaboration with BHV. In 2002, GlaxoSmithKline (GSK) received a license to use it. From 2002 to 2009, GSK carried out a significant clinical development program for the treatment of type-2 diabetes mellitus in various nations across the world and obesity in the UK. Remogliflozin etabonate's pharmacokinetics, pharmacodynamics, and clinical dose regimens were characterized in 18 Phase I and 2 Phase II investigations. Due to financial concerns, GSK stopped working on remogliflozin and sergliflozin, two further SGLT2 inhibitors that were licensed to the company, in 2009. Remogliflozin was commercially launched first in India by Glenmark in May 2019.
Glycine transporters (GlyTs) are plasmalemmal neurotransmitter transporters. They serve to terminate the signaling of glycine by mediating its reuptake from the synaptic cleft back into the presynaptic neurons. There are two glycine transporters: glycine transporter 1 (GlyT1) and glycine transporter 2 (GlyT2).
Milacemide (INN) is an MAO-B inhibitor and glycine prodrug. It has been studied for its effects on human memory and as a potential treatment for the symptoms of Alzheimer's disease. Early clinical trials did not show positive results however, and the drug is now abandoned and it is sold as a nonprescription drug or supplement. While milacemide is not an amino-acid, it acts similarly to glycine in the brain.

ORG-25935, also known as SCH-900435 is a synthetic drug developed by Organon International, which acts as a selective inhibitor of the glycine transporter GlyT-1. In animal tests it reduces alcohol consumption and has analgesic and anticonvulsant effects, but it has mainly been studied for its antipsychotic properties, and in human trials it was shown to effectively counteract the effects of the dissociative drug ketamine.

Bitopertin is a glycine reuptake inhibitor which was under development by Roche as an adjunct to antipsychotics for the treatment of persistent negative symptoms or suboptimally controlled positive symptoms associated with schizophrenia. Research into this indication has been largely halted as a result of disappointing trial results. As of 2024, it is under development for the management of erythropoietic protoporphyria.
Empagliflozin, sold under the brand name Jardiance, among others, is an antidiabetic medication used to improve glucose control in people with type 2 diabetes. It is taken by mouth.

CI-966 (developmental code name) is a central nervous system depressant acting as a GABA reuptake inhibitor, specifically a highly potent and selective blocker of the GABA transporter 1 (GAT-1) (IC50 = 0.26 μM), and hence indirect and non-selective GABA receptor full agonist. It was investigated as a potential anticonvulsant, anxiolytic, and neuroprotective therapeutic but was discontinued during clinical development due to the incidence of severe adverse effects at higher doses and hence was never marketed.
A glycine reuptake inhibitor (GRI) is a type of drug which inhibits the reuptake of the neurotransmitter glycine by blocking one or more of the glycine transporters (GlyTs). Examples of GRIs include bitopertin (RG1678), Org 24598, Org 25935, ALX-5407, and sarcosine, which are selective GlyT1 blockers, and Org 25543 and N-arachidonylglycine, which are selective GlyT2 blockers. Some weak and/or non-selective GlyT blockers include amoxapine and ethanol (alcohol).
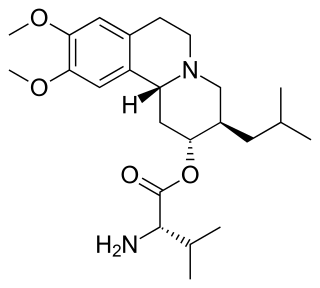
Valbenazine, sold under the brand name Ingrezza, is a medication used to treat tardive dyskinesia. It acts as a vesicular monoamine transporter 2 (VMAT2) inhibitor.

Pesampator is a positive allosteric modulator (PAM) of the AMPA receptor (AMPAR), an ionotropic glutamate receptor, which is under development by Pfizer for the treatment of cognitive symptoms in schizophrenia. It was also under development for the treatment of age-related sensorineural hearing loss, but development for this indication was terminated due to insufficient effectiveness. As of July 2018, pesampator is in phase II clinical trials for cognitive symptoms in schizophrenia.

Sotorasib, sold under the brand names Lumakras and Lumykras, is an anti-cancer medication used to treat non-small-cell lung cancer. It targets a specific mutation, G12C, in the protein K-Ras encoded by gene KRAS which is responsible for various forms of cancer. Sotorasib is an inhibitor of the RAS GTPase family.

Iclepertin is an investigational nootropic to enhance the cognition and functional capacity in schizophrenia developed by Boehringer Ingelheim. As of May 2020, it is in phase III of clinical trial under the code name CONNEX-3. BI 425809 is an inhibitor of glycine transporter 1 (Gly-T1) that in phase II improved cognition after 12 weeks in patients with schizophrenia. Doses of 10 mg and 25 mg showed the largest separation from placebo. If these encouraging results are confirmed in phase 3 trials, BI 425809 could provide an effective treatment for cognitive impairment associated with schizophrenia. Schizophrenia is characterized by abnormalities in glutamatergic pathways related to NMDA receptor hypofunction. Inhibition of GlyT1 on the presynaptic membrane or astrocytes is hypothesized to increase glycine levels within the synapse. The NMDA receptor function may be enhanced by increasing levels of its co-agonist, glycine, within the synaptic cleft, which may lead to improvements in cognitive function.
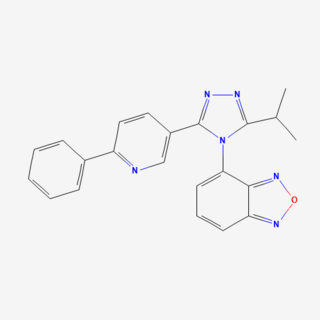
ASP2535 is an inhibitor of the type 1 glycine transporter. It could potentially be used in treatment of Alzheimer's disease and schizophrenia.

ORG-24598 is a selective inhibitor of the type 1 glycine transporter.















