
L-371,257 is a compound used in scientific research which acts as a selective antagonist of the oxytocin receptor with over 800x selectivity over the related vasopressin receptors. It was one of the first non-peptide oxytocin antagonists developed, and has good oral bioavailability, but poor penetration of the blood–brain barrier, which gives it good peripheral selectivity with few central side effects. Potential applications are likely to be in the treatment of premature labour.

PSB-10 is a drug which acts as a selective antagonist for the adenosine A3 receptor (ki value at human A3 receptor is 0.44 nM), with high selectivity over the other three adenosine receptor subtypes (ki values at human A1, A2A and A2B receptors are 4.1, 3.3 and 30 μM). Further pharmacological experiments in a [35S]GTPγS binding assay using hA3-CHO-cells indicated that PSB-10 acts as an inverse agonist (IC50 = 4 nM). It has been shown to produce antiinflammatory effects in animal studies. Simple xanthine derivatives such as caffeine and DPCPX have generally low affinity for the A3 subtype and must be extended by expanding the ring system and adding an aromatic group to give high A3 affinity and selectivity. The affinity towards adenosine A3 subtype was measured against the radioligand PSB-11.

UB-165 is a drug which acts as an agonist at neuronal nicotinic acetylcholine receptors being a full agonist of the α3β2 isoform and a partial agonist of the α4β2* isoform. It is used to study the role of this receptor subtype in the release of dopamine and noradrenaline in the brain, and has also been used as a lead compound to derive a number of other selective nicotinic receptor ligands.

Alniditan is a 5-HT1D receptor agonist with migraine-preventive effects.

Amentoflavone is a biflavonoid constituent of a number of plants including Ginkgo biloba, Chamaecyparis obtusa (hinoki), Hypericum perforatum and Xerophyta plicata.

Dextrallorphan (DXA) is an chemical of the morphinan class that is used in scientific research. It acts as a σ1 receptor agonist and NMDA receptor antagonist. It has no significant affinity for the σ2, μ-opioid, or δ-opioid receptor, or for the serotonin or norepinephrine transporter. As an NMDA receptor antagonist, in vivo, it is approximately twice as potent as dextromethorphan, and five-fold less potent than dextrorphan.

CECXG (3'-ethyl-LY-341,495) is a research drug which acts as a potent and selective antagonist for the group II metabotropic glutamate receptors (mGluR2/3), with reasonable selectivity for mGluR3. While it is some five times less potent than LY-341,495 at mGluR3, it has 38x higher affinity for mGluR3 over mGluR2, making it one of the few ligands available that is able to distinguish between these two closely related receptor subtypes.

CSP-2503 is a potent and selective 5-HT1A receptor agonist, 5-HT2A receptor antagonist, and 5-HT3 receptor antagonist of the phenylpiperazine class. First synthesized in 2003, it was designed based on computational models and QSAR studies. In rat studies, CSP-2503 has demonstrated anxiolytic effects, and thus has been suggested as a treatment for anxiety in humans with a multimodal mechanism of action.
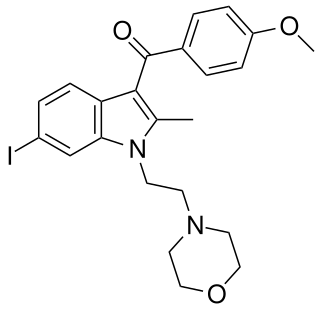
AM-630 (6-Iodopravadoline) is a drug that acts as a potent and selective inverse agonist for the cannabinoid receptor CB2, with a Ki of 32.1 nM at CB2 and 165x selectivity over CB1, at which it acted as a weak partial agonist. It is used in the study of CB2 mediated responses and has been used to investigate the possible role of CB2 receptors in the brain. AM-630 is significant as one of the first indole derived cannabinoid ligands substituted on the 6-position of the indole ring, a position that has subsequently been found to be important in determining affinity and efficacy at both the CB1 and CB2 receptors, and has led to the development of many related derivatives.

AM-1220 is a drug that acts as a potent and moderately selective agonist for the cannabinoid receptor CB1, with around 19 times selectivity for CB1 over the related CB2 receptor. It was originally invented in the early 1990s by a team led by Thomas D'Ambra at Sterling Winthrop, but has subsequently been researched by many others, most notably the team led by Alexandros Makriyannis at the University of Connecticut. The (piperidin-2-yl)methyl side chain of AM-1220 contains a stereocenter, so there are two enantiomers with quite different potency, the (R)-enantiomer having a Ki of 0.27 nM at CB1 while the (S)-enantiomer has a much weaker Ki of 217 nM.
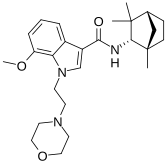
MN-25 (UR-12) is a drug invented by Bristol-Myers Squibb, that acts as a reasonably selective agonist of peripheral cannabinoid receptors. It has moderate affinity for CB2 receptors with a Ki of 11 nM, but 22x lower affinity for the psychoactive CB1 receptors with a Ki of 245 nM. The indole 2-methyl derivative has the ratio of affinities reversed however, with a Ki of 8 nM at CB1 and 29 nM at CB2, which contrasts with the usual trend of 2-methyl derivatives having increased selectivity for CB2 (cf. JWH-018 vs JWH-007, JWH-081 vs JWH-098).

Mazapertine (RWJ-37796) is an antipsychotic agent that was developed by Johnson & Johnson but never marketed. It exerts its pharmacological effect through affinity for dopamine D2, serotonin 5-HT1A, and α1-adrenergic receptors.

KM-233 is a synthetic cannabinoid drug which is a structural analog of Δ8-tetrahydrocannabinol (THC), the less active but more stable isomer of the active component of Cannabis. KM-233 differs from Δ8-THC by the pentyl side chain being replaced by a 1,1-dimethylbenzyl group. It has high binding affinity in vitro for both the CB1 and CB2 receptors, with a CB2 affinity of 0.91 nM and 13-fold selectivity over the CB1 receptor. In animal studies, it has been found to be a potential treatment for glioma, a form of brain tumor. Many related analogues are known where the 1,1-dimethylbenzyl group is substituted or replaced by other groups, with a fairly well established structure-activity relationship.

The vanilloids are compounds which possess a vanillyl group. They include vanillyl alcohol, vanillin, vanillic acid, acetovanillon, vanillylmandelic acid, homovanillic acid, capsaicin, etc. Isomers are the isovanilloids.
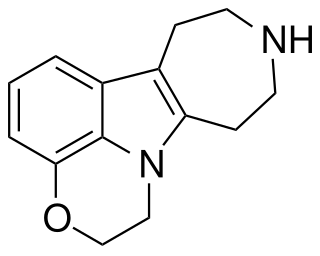
PHA-57378 is a drug which acts as an agonist at serotonin 5-HT2 receptors, having a binding affinity of 4.1 nM at the 5-HT2A subtype and 4.3 nM at 5-HT2C. It has anxiolytic effects in animal studies.
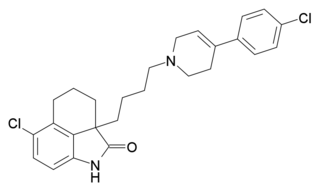
DR-4485 is a compound which acts as a potent and selective antagonist for the 5-HT7 receptor, with good oral bioavailability. It has been used to research the function of this still comparatively little studied serotonin receptor subtype.

ERX-11, also known as ERα coregulator-binding modulator-11, is a novel antiestrogen and experimental hormonal antineoplastic agent which is being researched for the potential treatment of estrogen receptor-positive breast cancer. It is not a competitive antagonist of the estrogen receptor (ER) like conventional antiestrogens such as tamoxifen or fulvestrant; instead of binding to the ligand-binding site of the ER, ERX-11 interacts with a different part of the ERα and blocks protein–protein interactions of the ERα with coregulators that are necessary for the receptor to act and regulate gene expression. It was designed to bind to the coregulator binding region of the ERα and inhibit the ERα/coactivator interaction, although its precise binding site and mode of action have yet to be fully elucidated and understood. Nonetheless, it is clear that ERX-11 binds within the AF-2 domain of the ERα.

NC 45-0095 is a synthetic nonsteroidal selective estrogen receptor modulator (SERM) which was under development by Novo Nordisk for the treatment of postmenopausal osteoporosis but was never marketed. It is a partial agonist of the estrogen receptor (IC50 (for binding inhibition) = 9.5 nM; EC50 = 13 nM) with mixed estrogenic and antiestrogenic activity, and shows full estrogenic activity in bone and uterus (Emax (relative to moxestrol, in Ishikawa endometrial cancer cell line) = 105%). The compound is a pyrroloindolizine derivative. Its development was discontinued by 2003.

SYM-2081 is a highly selective agonist for the kainate receptor. This potent agonist has nearly 3,000 fold- and 200-fold selectivity for kainate receptors over AMPA and NMDA receptors, respectively. Given its potency and selectivity, it is a useful ligand for studying the role of kainate receptors in the central nervous system.



















