
Allicin is an organosulfur compound obtained from garlic, a species in the family Alliaceae. It was first isolated and studied in the laboratory by Chester J. Cavallito and John Hays Bailey in 1944. When fresh garlic is chopped or crushed, the enzyme alliinase converts alliin into allicin, which is responsible for the aroma of fresh garlic. The allicin generated is unstable and quickly changes into a series of other sulfur-containing compounds such as diallyl disulfide. Allicin is part of a defense mechanism against attacks by pests on the garlic plant.

Cimetidine, sold under the brand name Tagamet among others, is a histamine H2 receptor antagonist that inhibits stomach acid production. It is mainly used in the treatment of heartburn and peptic ulcers.

Hydrogen bromide is the inorganic compound with the formula ‹See Tfd›HBr. It is a hydrogen halide consisting of hydrogen and bromine. A colorless gas, it dissolves in water, forming hydrobromic acid, which is saturated at 68.85% HBr by weight at room temperature. Aqueous solutions that are 47.6% HBr by mass form a constant-boiling azeotrope mixture that boils at 124.3 °C. Boiling less concentrated solutions releases H2O until the constant-boiling mixture composition is reached.
Myristic acid (IUPAC name: tetradecanoic acid) is a common saturated fatty acid with the molecular formula CH3(CH2)12COOH. Its salts and esters are commonly referred to as myristates or tetradecanoates. It is named after the binomial name for nutmeg (Myristica fragrans), from which it was first isolated in 1841 by Lyon Playfair.

Selenous acid (or selenious acid) is the chemical compound with the formula H2SeO3. Structurally, it is more accurately described by (HO)2SeO. It is the principal oxoacid of selenium; the other being selenic acid.

Methylergometrine, also known as methylergonovine and sold under the brand name Methergine, is a medication of the ergoline and lysergamide groups which is used as an oxytocic in obstetrics and in the treatment of migraine. It reportedly produces psychedelic effects similar to those of lysergic acid diethylamide (LSD) at high doses.
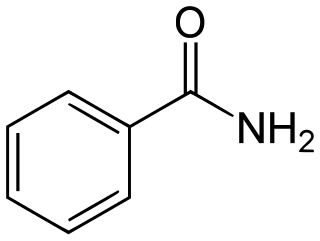
Benzamide is a white solid with the chemical formula of C6H5C(O)NH2. It is the simplest amide derivative of benzoic acid. It is slightly soluble in water, and soluble in many organic solvents. A number of substituted benzamides are commercial drugs: sulpiride, remoxipride, amisulpride, tiapride, sultopride, veralipride, aminohippuric acid, cisapride, imatinib, and procainamide.
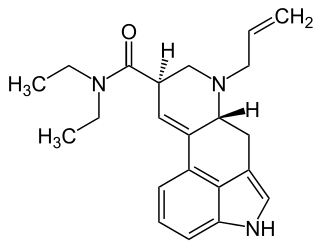
AL-LAD, also known as 6-allyl-6-nor-LSD, is a psychedelic drug and an analog of lysergic acid diethylamide (LSD). It is described by Alexander Shulgin in the book TiHKAL. It is synthesized starting from nor-LSD as a precursor, using allyl bromide as a reactant.

Potassium canrenoate or canrenoate potassium (USAN), also known as aldadiene kalium, the potassium salt of canrenoic acid, is an aldosterone antagonist of the spirolactone group. Like spironolactone, it is a prodrug, and is metabolized to active canrenone in the body.

Canrenone, sold under the brand names Contaren, Luvion, Phanurane, and Spiroletan, is a steroidal antimineralocorticoid of the spirolactone group related to spironolactone which is used as a diuretic in Europe, including in Italy and Belgium. It is also an important active metabolite of spironolactone, and partially accounts for its therapeutic effects.

γ-Amino-β-hydroxybutyric acid (GABOB), also known as β-hydroxy-γ-aminobutyric acid (β-hydroxy-GABA), and sold under the brand name Gamibetal among others, is an anticonvulsant which is used for the treatment of epilepsy in Europe, Japan, and Mexico. It is a GABA analogue, or an analogue of the neurotransmitter γ-aminobutyric acid (GABA), and has been found to be an endogenous metabolite of GABA.

Altrenogest, sold under the brand names Swinemate and Altren manufactured by Aurora Pharmaceutical and Regumate manufactured by Merck, is a progestin of the 19-nortestosterone group which is widely used in veterinary medicine to suppress or synchronize estrus in horses and pigs. It is available for veterinary use in both Europe and the United States.

Allylestrenol, sold under the brand names Gestanin and Turinal among others, is a progestin medication which is used to treat recurrent and threatened miscarriage and to prevent premature labor in pregnant women. However, except in the case of proven progesterone deficiency, its use for such purposes is no longer recommended. It is also used in Japan to treat benign prostatic hyperplasia (BPH) in men. The medication is used alone and is not formulated in combination with an estrogen. It is taken by mouth.

Promestriene (INN), also known as estradiol 3-propyl 17β-methyl diether, is a synthetic estrogen which is used topically in a 1% cream formulation for the treatment of vaginal atrophy in women. It is the 3-propyl and 17β-methyl diether of estradiol and does not appear to convert into estradiol in the body. Promestriene is minimally absorbed and appears to have negligible systemic estrogenic effect. The drug has been described as a tropic agent and antiseborrheic. It has not been found to be effective in the treatment of pattern hair loss or other conditions of cutaneous androgenization. Promestriene was first introduced in France in 1974 and has been marketed in 34 countries worldwide. It has been used in millions of women.
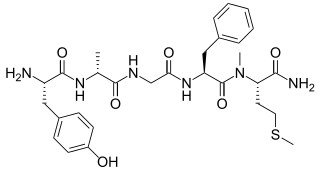
Metkefamide (INN; LY-127,623), or metkephamid acetate (USAN), but most frequently referred to simply as metkephamid, is a synthetic opioid pentapeptide and derivative of [Met]enkephalin with the amino acid sequence Tyr-D-Ala-Gly-Phe-(N-Me)-Met-NH2. It behaves as a potent agonist of the δ- and μ-opioid receptors with roughly equipotent affinity, and also has similarly high affinity as well as subtype-selectivity for the κ3-opioid receptor.

Diacetylnalorphine (BAN), also known as O3,O6-diacetyl-N-allyl-normorphine, is an opioid drug described as an analgesic and antidote which was never marketed. It is the 3,6-diacetyl ester of nalorphine, and therefore the heroin analogue of nalorphine. Diacetylnalorphine may behave as a prodrug to nalorphine, similarly to the cases of heroin (diacetylmorphine) to morphine and diacetyldihydromorphine to dihydromorphine.

Epipregnanolone, also known as 3β-hydroxy-5β-pregnan-20-one, 3β,5β-tetrahydroprogesterone, or 3β,5β-THP, is an endogenous neurosteroid. It acts as a negative allosteric modulator of the GABAA receptor and reverses the effects of potentiators like allopregnanolone. Epipregnanolone is biosynthesized from progesterone by the actions of 5β-reductase and 3β-hydroxysteroid dehydrogenase, with 5β-dihydroprogesterone as the intermediate in this two-step transformation.

Hydroxyprogesterone heptanoate (OHPH), also known as hydroxyprogesterone enanthate (OHPE) and sold under the brand names H.O.P., Lutogil A.P., and Lutogyl A.P. among others, is a progestin medication used for progestogenic indications. It has been formulated both alone and in together with estrogens, androgens/anabolic steroids, and other progestogens in several combination preparations. OHPH is given by injection into muscle at regular intervals.

Doisynolic acid is a synthetic, nonsteroidal, orally active estrogen that was never marketed. The reaction of estradiol or estrone with potassium hydroxide, a strong base, results in doisynolic acid as a degradation product, which retains high estrogenic activity, and this reaction was how the drug was discovered, in the late 1930s. The drug is a highly active and potent estrogen by the oral or subcutaneous route. The reaction of equilenin or dihydroequilenin with potassium hydroxide was also found to produce bisdehydrodoisynolic acid, the levorotatory isomer of which is an estrogen with an "astonishingly" high degree of potency, while the dextrorotatory isomer is inactive. Doisynolic acid was named after Edward Adelbert Doisy, a pioneer in the field of estrogen research and one of the discoverers of estrone.

Allenestrol, or allenoestrol, also known as α,α-dimethyl-β-ethylallenolic acid or as methallenestrilphenol, is a synthetic, nonsteroidal estrogen and a derivative of allenolic acid that was never marketed. A methyl ether of allenestrol, methallenestril (methallenestrol), is also an estrogen, but, in contrast to allenestrol, has been marketed.



















