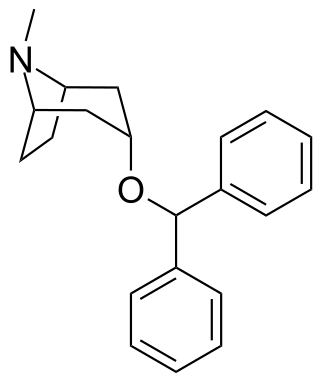
Benzatropine (INN), known as benztropine in the United States and Japan, is a medication used to treat movement disorders like parkinsonism and dystonia, as well as extrapyramidal side effects of antipsychotics, including akathisia. It is not useful for tardive dyskinesia. It is taken by mouth or by injection into a vein or muscle. Benefits are seen within two hours and last for up to ten hours.
An international nonproprietary name (INN) is an official generic and nonproprietary name given to a pharmaceutical drug or an active ingredient. INNs are intended to make communication more precise by providing a unique standard name for each active ingredient, to avoid prescribing errors. The INN system has been coordinated by the World Health Organization (WHO) since 1953.

Etoperidone, associated with several brand names, is an atypical antidepressant which was developed in the 1970s and either is no longer marketed or was never marketed. It is a phenylpiperazine related to trazodone and nefazodone in chemical structure and is a serotonin antagonist and reuptake inhibitor (SARI) similarly to them.

Cyprazepam is a drug which is a sedative-hypnotic benzodiazepine derivative. It has anxiolytic properties, and presumably also has hypnotic, skeletal muscle relaxant, anticonvulsant and amnestic properties.
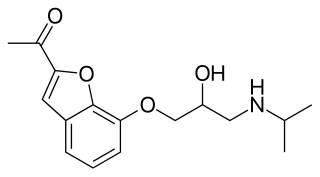
Befunolol (INN) is a beta blocker with intrinsic sympathomimetic activity used in the management of open-angle glaucoma. It also acts as a β adrenoreceptor partial agonist. Befunolol was introduced in Japan in 1983 by Kakenyaku Kako Co. under the trade name Bentos.

Oxaflozane (INN) is an antidepressant and anxiolytic drug that was introduced by Solvay in France in 1982 for the treatment of depression but has since been discontinued. It is a prodrug of flumexadol, which is reported to act as an agonist of the serotonin 5-HT1A and 5-HT2C receptors and, to a much lesser extent, of the 5-HT2A receptor. In addition to its serotonergic properties, oxaflozane may also produce anticholinergic side effects at high doses, namely in overdose.

Homarylamine is an antitussive (anti-cough) drug which was patented in 1956 by Merck & Co., but has never been used medically as such.
Adaprolol is a beta blocker.

Xibenolol is a beta blocker.

Difemetorex, also known as diphemethoxidine, is a stimulant drug of the piperidine class which was used as an appetite suppressant, but produced intolerable side effects such as insomnia which limited its clinical use. It was introduced in France by Ciba-Geigy in 1966 but is now no longer marketed.

1-Phenylpiperazine is a simple chemical compound featuring a phenyl group bound to a piperazine ring. The suffix ‘-piprazole’ is sometimes used in the names of drugs to indicate they belong to this class.
Drug nomenclature is the systematic naming of drugs, especially pharmaceutical drugs. In the majority of circumstances, drugs have 3 types of names: chemical names, the most important of which is the IUPAC name; generic or nonproprietary names, the most important of which are international nonproprietary names (INNs); and trade names, which are brand names. Under the INN system, generic names for drugs are constructed out of affixes and stems that classify the drugs into useful categories while keeping related names distinguishable. A marketed drug might also have a company code or compound code.
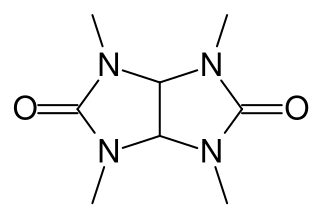
Temgicoluril (INN), also known as tetramethylglycoluril and sold under the brand names Adaptol and Mebicar, is an anxiolytic medication produced by Latvian pharmaceutical company Olainfarm and sold in Latvia and Russia.

Suntinorexton (INN) is an experimental orexin receptor agonist. It acts as a selective agonist of the orexin OX2 receptor and was described in 2019 in a patent by Takeda Pharmaceutical Company.

Firazorexton (INN) is an experimental orexin 2 (OX2) receptor agonist first described in a 2019 patent filed by Takeda Pharmaceutical Company.












