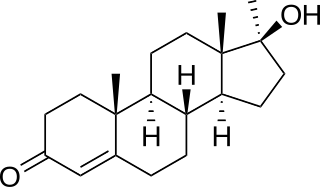
Methyltestosterone, sold under the brand names Android, Metandren, and Testred among others, is an androgen and anabolic steroid (AAS) medication which is used in the treatment of low testosterone levels in men, delayed puberty in boys, at low doses as a component of menopausal hormone therapy for menopausal symptoms like hot flashes, osteoporosis, and low sexual desire in women, and to treat breast cancer in women. It is taken by mouth or held in the cheek or under the tongue.

Nandrolone, also known as 19-nortestosterone, is an endogenous androgen. It is also an anabolic steroid (AAS) which is medically used in the form of esters such as nandrolone decanoate and nandrolone phenylpropionate. Nandrolone esters are used in the treatment of anemias, cachexia, osteoporosis, breast cancer, and for other indications. They are now used by oral administration or instead are given by injection into muscle or fat.
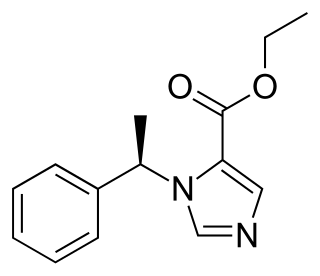
Etomidate is a short-acting intravenous anaesthetic agent used for the induction of general anaesthesia and sedation for short procedures such as reduction of dislocated joints, tracheal intubation, cardioversion and electroconvulsive therapy. It was developed at Janssen Pharmaceutica in 1964 and was introduced as an intravenous agent in 1972 in Europe and in 1983 in the United States.

Dehydroepiandrosterone sulfate, abbreviated as DHEA sulfate or DHEA-S, also known as androstenolone sulfate, is an endogenous androstane steroid that is produced by the adrenal cortex. It is the 3β-sulfate ester and a metabolite of dehydroepiandrosterone (DHEA) and circulates in far greater relative concentrations than DHEA. The steroid is hormonally inert and is instead an important neurosteroid and neurotrophin.

Medroxyprogesterone (MP), is a progestin which is not used medically. A derivative, medroxyprogesterone acetate (MPA), is used as a medication in humans, and is far more widely known in comparison. Medroxyprogesterone is sometimes used as a synonym for medroxyprogesterone acetate, and what is almost always being referred to when the term is used is MPA and not medroxyprogesterone.

Gestonorone caproate, also known as gestronol hexanoate or norhydroxyprogesterone caproate and sold under the brand names Depostat and Primostat, is a progestin medication which is used in the treatment of enlarged prostate and cancer of the endometrium. It is given by injection into muscle typically once a week.

Allopregnanolone is a naturally occurring neurosteroid which is made in the body from the hormone progesterone. As a medication, allopregnanolone is referred to as brexanolone, sold under the brand name Zulresso, and used to treat postpartum depression. It is given by injection into a vein.
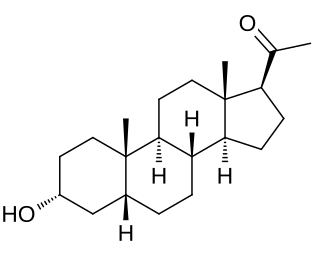
Pregnanolone, also known as eltanolone, is an endogenous inhibitory neurosteroid which is produced in the body from progesterone. It is closely related to allopregnanolone, which has similar properties.
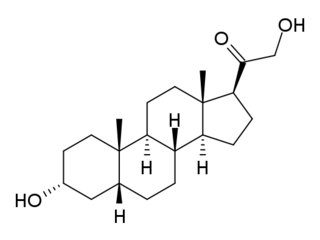
Tetrahydrodeoxycorticosterone, also referred to as allotetrahydrocorticosterone, is an endogenous neurosteroid. It is synthesized from the adrenal hormone deoxycorticosterone by the action of two enzymes, 5α-reductase type I and 3α-hydroxysteroid dehydrogenase. THDOC is a potent positive allosteric modulator of the GABAA receptor, and has sedative, anxiolytic and anticonvulsant effects. Changes in the normal levels of this steroid particularly during pregnancy and menstruation may be involved in some types of epilepsy and premenstrual syndrome, as well as stress, anxiety and depression.

Normethandrone, also known as methylestrenolone or methylnortestosterone and sold under the brand name Metalutin among others, is a progestin and androgen/anabolic steroid (AAS) medication which is used in combination with an estrogen in the treatment of amenorrhea and menopausal symptoms in women. It is taken by mouth.

Trimegestone, sold under the brand names Ondeva and Totelle among others, is a progestin medication which is used in menopausal hormone therapy and in the prevention of postmenopausal osteoporosis. It was also under development for use in birth control pills to prevent pregnancy, but ultimately was not marketed for this purpose. The medication is available alone or in combination with an estrogen. It is taken by mouth.

20α-Dihydroprogesterone (20α-DHP), also known as 20α-hydroxyprogesterone (20α-OHP), is a naturally occurring, endogenous progestogen. It is a metabolite of progesterone, formed by the 20α-hydroxysteroid dehydrogenases (20α-HSDs) AKR1C1, AKR1C2, and AKR1C3 and the 17β-hydroxysteroid dehydrogenase (17β-HSD) HSD17B1. 20α-DHP can be transformed back into progesterone by 20α-HSDs and by the 17β-HSD HSD17B2. HSD17B2 is expressed in the human endometrium and cervix among other tissues. In animal studies, 20α-DHP has been found to be selectively taken up into and retained in target tissues such as the uterus, brain, and skeletal muscle.

Methylestradiol, sold under the brand names Ginecosid, Ginecoside, Mediol, and Renodiol, is an estrogen medication which is used in the treatment of menopausal symptoms. It is formulated in combination with normethandrone, a progestin and androgen/anabolic steroid medication. Methylestradiol is taken by mouth.

Anagestone acetate, sold under the brand names Anatropin and Neo-Novum, is a progestin medication which was withdrawn from medical use due to carcinogenicity observed in animal studies.
A steroidogenesis inhibitor, also known as a steroid biosynthesis inhibitor, is a type of drug which inhibits one or more of the enzymes that are involved in the process of steroidogenesis, the biosynthesis of endogenous steroids and steroid hormones. They may inhibit the production of cholesterol and other sterols, sex steroids such as androgens, estrogens, and progestogens, corticosteroids such as glucocorticoids and mineralocorticoids, and neurosteroids. They are used in the treatment of a variety of medical conditions that depend on endogenous steroids.

Chloroethynylnorgestrel is a steroidal progestin of the 19-nortestosterone group related to norgestrel that was investigated as an oral contraceptive in the 1970s but was never marketed.

BOMT, also known by its developmental code name Ro 7-2340 and as 6α-bromo-4-oxa-17α-methyl-5α-dihydrotestosterone, is a synthetic steroidal antiandrogen which was first produced in 1970 and was never marketed for medical use. It is the 6α-brominated, 4-oxygenated, and 17α-methylated derivative of the androgen dihydrotestosterone (DHT). Along with benorterone, cyproterone, and flutamide, BOMT was among the earliest antiandrogens to be developed and extensively studied, although it is less well-documented in comparison to the others. BOMT has been investigated clinically in the treatment of benign prostatic hyperplasia, though development for this use did not continue. There was also interest in BOMT for the potential applications of acne, pattern hair loss, and possibly prostate cancer, but it was not developed for these indications either.
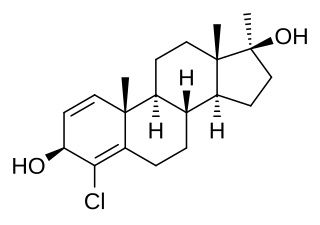
Chlorodehydromethylandrostenediol (CDMA), also known as 4-chloro-17α-methylandrost-1,4-diene-3β,17β-diol, is a synthetic, orally active anabolic-androgenic steroid (AAS) and a 17α-alkylated derivative of 4-androstenediol that was never marketed. It was first encountered in 2005 when it was introduced as a "dietary supplement" and putative prohormone under the name Halodrol-50 by industry veteran, Bruce Kneller while working with the dietary supplement company, Gaspari Nutrition. The drug was the subject of a scathing and highly critical article by The Washington Post in November 2006. CDMA was voluntarily discontinued by Gaspari Nutrition in mid-2006, likely fearing government sanctions if it continued to sell the product. During the brief period of time that CDMA was sold online, it was an extremely well-selling product; its total sales are estimated to have been greater than twenty five million dollars, and by some estimates, CDMA may have been the best-selling hormonal product ever sold "over-the-counter" in the United States.CDMA continued to be sold online until the 2014 prohormone ban as generic versions known as clones.
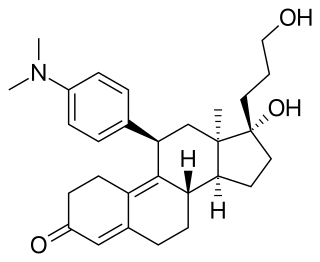
Onapristone is a synthetic and steroidal antiprogestogen with additional antiglucocorticoid activity which was developed by Schering and described in 1984 but was never marketed. It is a silent antagonist of the progesterone receptor (PR), in contrast to the related antiprogestogen mifepristone. Moreover, compared to mifepristone, onapristone has reduced antiglucocorticoid activity, shows little antiandrogenic activity, and has 10- to 30-fold greater potency as an antiprogestogen. The medication was under development for clinical use, for instance in the treatment of breast cancer and as an endometrial contraceptive, but was discontinued during phase III clinical trials in 1995 due to findings that liver function abnormalities developed in a majority patients.

17α-Allyl-19-nortestosterone, also known as 3-ketoallylestrenol or as 17α-allylestr-4-en-17β-ol-3-one, is a progestin which was never marketed. It is a combined derivative of the anabolic–androgenic steroid and progestogen nandrolone (19-nortestosterone) and the antiandrogen allyltestosterone (17α-allyltestosterone). The drug is a major active metabolite of allylestrenol, which is thought to be a prodrug of 17α-allyl-19-nortestosterone.



















