 | |
 | |
| Clinical data | |
|---|---|
| Dependence liability | Very high |
| Routes of administration | Oral |
| Legal status | |
| Legal status | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.428.803 |
| Chemical and physical data | |
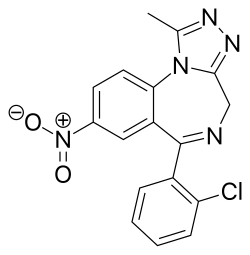
| Formula | C17H12ClN5O2 |
| Molar mass | 353.77 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Clonazolam (also known as clonitrazolam) is a drug of the triazolobenzodiazepine (TBZD) class, which are benzodiazepines (BZDs) fused with a triazole ring. Although little research has been done about its effects and metabolism, it is sold online as a designer drug. [2] [3] [4] [5] [6]
Contents
The synthesis of clonazolam was first reported in 1971 and the drug was described as the most active compound in the series tested. [7]
Depending on dose consumed, clonazolam may pose comparatively higher risk than other designer benzodiazepines due to its ability to produce strong sedation and amnesia at doses as small as 0.5 mg. [2] [8]