
Bromazepam, sold under many brand names, is a benzodiazepine. It is mainly an anti-anxiety agent with similar side effects to diazepam. In addition to being used to treat anxiety or panic states, bromazepam may be used as a premedicant prior to minor surgery. Bromazepam typically comes in doses of 3 mg and 6 mg tablets.

Quazepam, sold under the brand name Doral among others, is a relatively long-acting benzodiazepine derivative drug developed by the Schering Corporation in the 1970s. Quazepam is used for the treatment of insomnia, including sleep induction and sleep maintenance. Quazepam induces impairment of motor function and has relatively selective hypnotic and anticonvulsant properties with considerably less overdose potential than other benzodiazepines. Quazepam is an effective hypnotic which induces and maintains sleep without disruption of the sleep architecture.
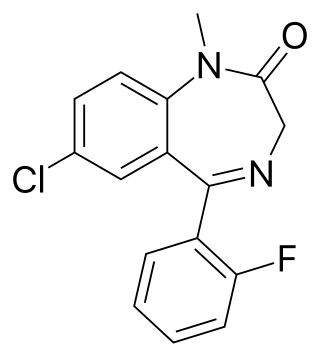
Fludiazepam, marketed under the brand name Erispan (エリスパン) is a potent benzodiazepine and 2ʹ-fluoro derivative of diazepam, originally developed by Hoffmann-La Roche in the 1960s. It is marketed in Japan and Taiwan. It exerts its pharmacological properties via enhancement of GABAergic inhibition. Fludiazepam has 4 times more binding affinity for benzodiazepine receptors than diazepam. It possesses anxiolytic, anticonvulsant, sedative, hypnotic and skeletal muscle relaxant properties. Fludiazepam has been used recreationally.

QH-II-66 (QH-ii-066) is a sedative drug which is a benzodiazepine derivative. It produces some of the same effects as other benzodiazepines, but is much more selective than most other drugs of this class and so produces somewhat less sedation and ataxia than other related drugs such as diazepam and triazolam, although it still retains anticonvulsant effects.

Meclonazepam ((S)-3-methylclonazepam) was discovered by a team at Hoffmann-La Roche in the 1970s and is a drug which is a benzodiazepine derivative similar in structure to clonazepam. It has sedative and anxiolytic actions like those of other benzodiazepines, and also has anti-parasitic effects against the parasitic worm Schistosoma mansoni.

JM-1232 is a sedative and hypnotic drug being researched as a potential anesthetic. It has similar effects to sedative-hypnotic benzodiazepine drugs, but is structurally distinct and so is classed as a nonbenzodiazepine hypnotic. It was developed by a team at Maruishi Pharmaceutica.

In pharmacology, GABAA receptor positive allosteric modulators, also known as GABAkines or GABAA receptor potentiators, are positive allosteric modulator (PAM) molecules that increase the activity of the GABAA receptor protein in the vertebrate central nervous system.
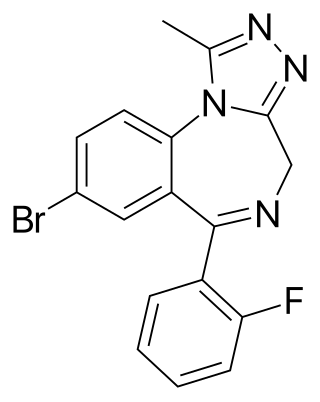
Flubromazolam (JYI-73) is a triazolobenzodiazepine (TBZD), which are benzodiazepine (BZD) derivatives. Flubromazolam is reputed to be highly potent, and concerns have been raised that clonazolam and flubromazolam in particular may pose comparatively higher risks than other designer benzodiazepines, due to their ability to produce strong sedation and amnesia at oral doses of as little as 0.5 mg. Life-threatening adverse reactions have been observed at doses of only 3 mg of flubromazolam.

Desmethylflunitrazepam (also known as norflunitrazepam, Ro05-4435 and fonazepam) is a benzodiazepine that is a metabolite of flunitrazepam and has been sold online as a designer drug. It has an IC50 value of 1.499 nM for the GABAA receptor.

Flunitrazolam is a triazolobenzodiazepine (TBZD), which are benzodiazepine (BZD) derivatives, that has been sold online as a designer drug, and is a potent hypnotic and sedative drug similar to related compounds such as flunitrazepam, clonazolam and flubromazolam. It was first definitively identified and reported to the EMCDDA Early Warning System, by an analytical laboratory in Germany in October 2016, and had not been described in the scientific or patent literature before this. It is the triazole analogue of Flunitrazepam (Rohypnol). The addition of the triazole ring to the scaffold increases potency significantly, this is evident as flunitrazolam is reported anecdotally to be active in the microgram level. It is active at 0.1 mg.
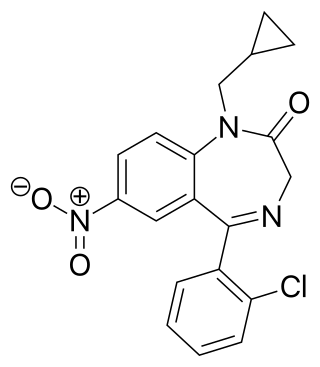
Cloniprazepam is a benzodiazepine derivative and a prodrug of clonazepam, 7-aminoclonazepam, and other metabolites.

GL-II-73 (GL-ii-073) is a benzodiazepine derivative related in chemical structure to compounds such as midazolam and adinazolam. It is described as an α5 preferring positive allosteric modulator of the benzodiazepine site of GABAA receptors, with weaker activity at α2 and α3 and no significant affinity for the α1 subtype. In animal tests it was found to produce effects consistent with antidepressant, anxiolytic and nootropic actions.

Fluclotizolam is a thienotriazolodiazepine derivative which was first synthesised in 1979, but was never marketed. It has subsequently been sold as a designer drug, first being definitively identified in 2017.

Ro09-9212 is a thienodiazepine derivative with sedative and anxiolytic effects, which has been sold as a designer drug.

Ro07-9749 is a benzodiazepine derivative with sedative and anxiolytic effects, which has been used as an internal standard in the analysis of other benzodiazepines, and also sold as a designer drug.

Fluadinazolam is a benzodiazepine derivative developed in 1973, with sedative and anxiolytic effects. It is a derivative of the never commercially marketed benzodiazepine adinazolam and has similarly been sold as a designer drug.
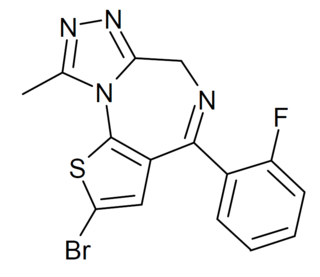
Flubrotizolam is a thienotriazolodiazepine derivative with potent sedative and anxiolytic effects, which has been sold as a designer drug.

Ro20-8065 (8-Chloronorflurazepam) is a benzodiazepine derivative with anticonvulsant and muscle relaxant effects, which has been sold as a designer drug.

Ro07-5220 (6'-Chlorodiclazepam) is a benzodiazepine derivative with sedative, anxiolytic, anticonvulsant and muscle relaxant effects, which has been sold as a designer drug.



















