An induced coma – also known as a medically induced coma (MIC), barbiturate-induced coma, or drug-induced coma – is a temporary coma brought on by a controlled dose of an anesthetic drug, often a barbiturate such as pentobarbital or thiopental. Other intravenous anesthetic drugs such as midazolam or propofol may be used.

Phenobarbital, also known as phenobarbitone or phenobarb, sold under the brand name Luminal among others, is a medication of the barbiturate type. It is recommended by the World Health Organization (WHO) for the treatment of certain types of epilepsy in developing countries. In the developed world, it is commonly used to treat seizures in young children, while other medications are generally used in older children and adults. In developed countries it is used for veterinary purposes. It may be used intravenously, injected into a muscle, or taken by mouth. The injectable form may be used to treat status epilepticus. Phenobarbital is occasionally used to treat trouble sleeping, anxiety, and drug withdrawal and to help with surgery. It usually begins working within five minutes when used intravenously and half an hour when administered by mouth. Its effects last for between four hours and two days.

Butabarbital is a prescription barbiturate sleep aid and anxiety medication. Butabarbital has a particularly fast onset of effects and short duration of action compared to other barbiturates, which makes it useful for certain applications such as treating severe insomnia, relieving general anxiety and relieving anxiety before surgical procedures; however it is also relatively dangerous particularly when combined with alcohol, and so is now rarely used, although it is still prescribed in some Eastern European and South American countries. Its intermediate duration of action gives butabarbital an abuse potential slightly lower than secobarbital. Butabarbital can be hydrolyzed to Valnoctamide.
Methylphenobarbital (INN), also known as mephobarbital and mephobarbitone (BAN), marketed under brand names such as Mebaral, Mephyltaletten, Phemiton, and Prominal, is a drug which is a barbiturate derivative and is used primarily as an anticonvulsant, but also as a sedative and anxiolytic. It is the N-methylated analogue of phenobarbital and has similar indications, therapeutic value, and tolerability.
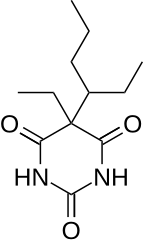
Heptabarb, also known as heptabarbitone (BAN) or heptabarbital, is a sedative and hypnotic drug of the barbiturate family. It was used in Europe for the treatment of insomnia from the 1950s onwards, but has since been discontinued.
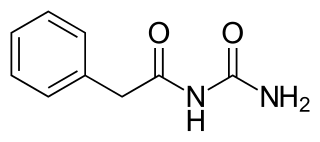
Phenacemide, also known as phenylacetylurea, is an anticonvulsant of the ureide (acetylurea) class. It is a congener and ring-opened analogue of phenytoin, and is structurally related to the barbiturates and to other hydantoins. Phenacemide was introduced in 1949 for the treatment of epilepsy, but was eventually withdrawn due to toxicity.

Allobarbital, also known as allobarbitone and branded as Dial, Cibalgine, or Dial-Ciba, is a barbiturate derivative invented in 1912 by Ernst Preiswerk and Ernst Grether working for CIBA. It was used primarily as an anticonvulsant although it has now largely been replaced by newer drugs with improved safety profiles. Other uses for allobarbital included as an adjutant to boost the activity of analgesic drugs, and use in the treatment of insomnia and anxiety.
Febarbamate, also known as phenobamate, is an anxiolytic and tranquilizer of the barbiturate and carbamate families which is used in Europe by itself and as part of a combination drug formulation called tetrabamate.
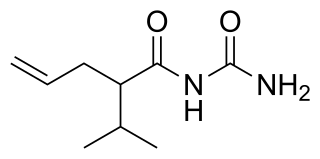
Apronal, or apronalide, also known as allylisopropylacetylurea or allylisopropylacetylcarbamide, is a hypnotic/sedative drug of the ureide (acylurea) group synthesized in 1926 by Hoffmann-La Roche that is no longer used except in Japan. Though it is not a barbiturate, apronalide is similar in structure to the barbiturates. In accordance, it is similar in action to the barbiturates, although considerably milder in comparison. Upon the finding that it caused patients to develop thrombocytopenic purpura, apronalide was withdrawn from clinical use.

Etamivan is a respiratory stimulant drug related to nikethamide. It was mainly used in the treatment of barbiturate overdose and chronic obstructive pulmonary disease, but has now largely fallen into disuse.

Embutramide is a potent opioid analgesic and sedative drug that is structurally related to methadone. It was developed by Hoechst A.G. in 1958 and was investigated as a general anesthetic agent, but was found to have a very narrow therapeutic window, with a 50 mg/kg dose producing effective sedation and a 75 mg/kg dose being fatal. Along with strong sedative effects, embutramide also produces respiratory depression and ventricular arrhythmia. Because of these properties, it was never adopted for medical use as an anesthetic as it was considered too dangerous for this purpose. Instead it is used for euthanasia in veterinary medicine, mainly for the euthanization of dogs.
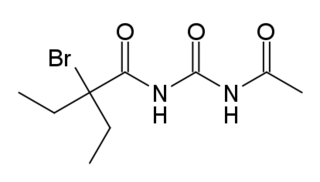
Acecarbromal (INN), also known as acetylcarbromal and acetyladalin, is a hypnotic and sedative drug of the ureide (acylurea) group discovered by Bayer in 1917 that was formerly marketed in the United States and Europe. It is also used in combination with extract of quebracho and vitamin E as a treatment for erectile dysfunction under the brand name Afrodor in Europe. Acecarbromal is structurally related to the barbiturates, which are basically cyclized ureas. Prolonged use is not recommended as it can cause bromine poisoning.

Barbiturates are a class of depressant drugs that are chemically derived from barbituric acid. They are effective when used medically as anxiolytics, hypnotics, and anticonvulsants, but have physical and psychological addiction potential as well as overdose potential among other possible adverse effects. They have been used recreationally for their anxiolytic and sedative effects, and are thus controlled in most countries due to the risks associated with such use.

Fenpentadiol (INN), also known as phenpentanediol, is a drug described as a tranquilizer and antidepressant that was formerly marketed in Europe. It also has stimulant, sedative, and anxiolytic effects, with the latter two occurring only at higher doses.
Barbiturate dependence develops with regular use of barbiturates. This in turn may lead to a need for increasing doses of the drug to get the original desired pharmacological or therapeutic effect. Barbiturate use can lead to both addiction and physical dependence, and as such they have a high potential for excess or non-medical use, however, it does not affect all users. Management of barbiturate dependence involves considering the affected person's age, comorbidity and the pharmacological pathways of barbiturates.
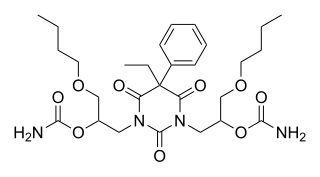
Difebarbamate (INN) is a tranquilizer of the barbiturate and carbamate families which is used in Europe as a component of a combination drug formulation referred to as tetrabamate.

Thiotetrabarbital is a drug which is a short-acting barbiturate derivative that is used as an anesthetic. It has been used in veterinary medicine.
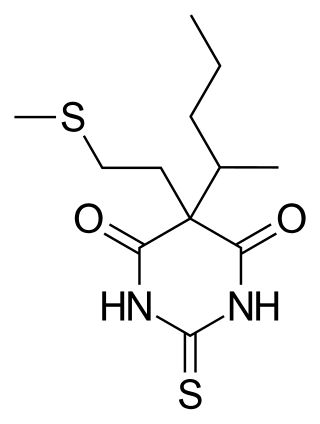
Methitural, or methitural sodium, also known as methioturiate, is a barbiturate derivative which was marketed in the 1950s in Europe as an ultra-short-acting intravenous anesthetic.

Buthalital sodium, or buthalitone sodium (BAN), is a barbiturate derivative which was under development as a short-acting anesthetic. However, development was discontinued, perhaps due to its extremely rapid elimination rate, and buthalital sodium was never marketed.

Etodroxizine (INN) is a first-generation antihistamine of the diphenylmethylpiperazine group which is used as a sedative/hypnotic drug in Europe and South Africa.