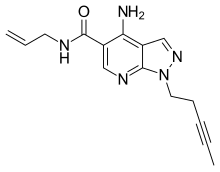
Alpidem (Ananxyl) is an anxiolytic drug from the imidazopyridine family, related to the more well known sleeping medication zolpidem. Unlike zolpidem however, alpidem does not produce sedative effects at normal doses, and is instead used specifically for the treatment of anxiety.

Pinazepam is a benzodiazepine drug. It possesses anxiolytic, anticonvulsant, sedative and skeletal muscle relaxant properties.

Gidazepam, also known as hydazepam or hidazepam, is a drug which is an atypical benzodiazepine derivative, developed in the Soviet Union. It is a selectively anxiolytic benzodiazepine. It also has therapeutic value in the management of certain cardiovascular disorders. It can also be used for a treatment to giddiness.
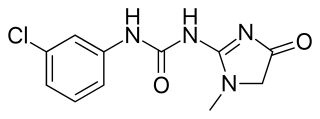
Fenobam is an imidazole derivative developed by McNeil Laboratories in the late 1970s as a novel anxiolytic drug with an at-the-time-unidentified molecular target in the brain. Subsequently, it was determined that fenobam acts as a potent and selective negative allosteric modulator of the metabotropic glutamate receptor subtype mGluR5, and it has been used as a lead compound for the development of a range of newer mGluR5 antagonists.

Etifoxine is an anxiolytic and anticonvulsant drug developed by Hoechst in the 1960s. It is sold in approximately 40 countries for anxiety disorders, without the sedation and ataxia associated with benzodiazepine drugs. It has similar anxiolytic effects to benzodiazepine drugs, but is structurally distinct, although it has structural elements in common with them. Studies suggest is as effective as lorazepam as an anxiolytic, but has fewer side effects. Etifoxine is not approved by the U.S. Food and Drug Administration or the European Medicines Agency.
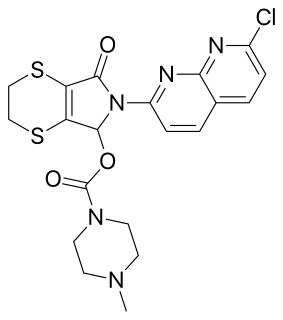
Suriclone (Suril) is a sedative and anxiolytic drug in the cyclopyrrolone family of drugs. Other cyclopyrrolone drugs include zopiclone and pagoclone.

Panadiplon (U-78875) is an anxiolytic drug with a novel chemical structure that is not closely related to other drugs of this type. It has a similar pharmacological profile to the benzodiazepine family of drugs, but with mainly anxiolytic properties and relatively little sedative or amnestic effect, and so is classified as a nonbenzodiazepine anxiolytic.

Pazinaclone (DN-2327) is a sedative and anxiolytic drug in the cyclopyrrolone family of drugs. Some other cyclopyrrolone drugs include zopiclone and eszopiclone.
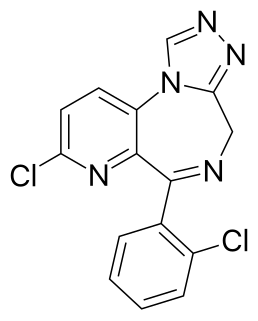
Zapizolam is a pyridodiazepine drug, which is a benzodiazepine analog of pyridotriazolodiazepine group. It has sedative and anxiolytic effects similar to those produced by benzodiazepine derivatives, and has been sold illicitly as a designer drug.
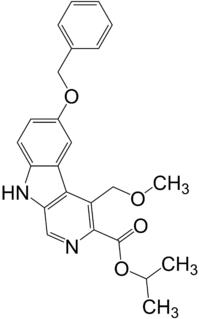
Abecarnil (ZK-112,119) is an anxiolytic drug from the β-Carboline family. It is one of a relatively recently developed class of medicines known as the nonbenzodiazepines, which have similar effects to the older benzodiazepine group, but with quite different chemical structures. It is a partial agonist acting selectively at the benzodiazepine site of the GABAA receptor.

Pipequaline (INN) is an anxiolytic drug that was never marketed. It possesses a novel chemical structure that is not closely related to other drugs of this type. The drug has a similar pharmacological profile to the benzodiazepine family of drugs, but with mainly anxiolytic properties and very little sedative, amnestic or anticonvulsant effects, and so is classified as a nonbenzodiazepine anxiolytic.

Uldazepam is a drug which is a benzodiazepine derivative. It has sedative and anxiolytic effects similar to those of other benzodiazepines.

Triflubazam is a drug which is a 1,5-benzodiazepine derivative, related to clobazam. It has sedative and anxiolytic effects, with a long half-life and duration of action.

SL651498 is an anxiolytic and anticonvulsant drug used in scientific research, with a chemical structure most closely related to β-carboline derivatives such as abecarnil and gedocarnil. It has similar effects to benzodiazepine drugs, but is structurally distinct and so is classed as a nonbenzodiazepine anxiolytic.

Etazolate (SQ-20,009, EHT-0202) is an anxiolytic drug which is a pyrazolopyridine derivative and has unique pharmacological properties. It acts as a positive allosteric modulator of the GABAA receptor at the barbiturate binding site, as an adenosine antagonist of the A1 and A2 subtypes, and as a phosphodiesterase inhibitor selective for the PDE4 isoform. It is currently in clinical trials for the treatment of Alzheimer's disease.

Tracazolate (ICI-136,753) is an anxiolytic drug which is used in scientific research. It is a pyrazolopyridine derivative, most closely related to pyrazolopyrimidine drugs such as zaleplon, and is one of a structurally diverse group of drugs known as the nonbenzodiazepines which act at the same receptor targets as benzodiazepines but have distinct chemical structures.

ELB-139 (LS-191,811) is an anxiolytic drug with a novel chemical structure, which is used in scientific research. It has similar effects to benzodiazepine drugs, but is structurally distinct and so is classed as a nonbenzodiazepine anxiolytic.
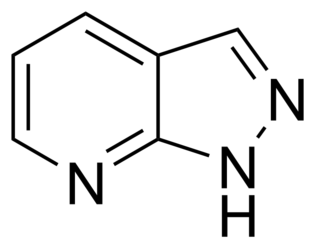
The pyrazolopyridines are a group of drugs investigated as anxiolytics which act as positive allosteric modulators of the GABAA receptor via the barbiturate binding site. They include the following compounds:

Cartazolate (SQ-65,396) is a drug of the pyrazolopyridine class. It acts as a GABAA receptor positive allosteric modulator at the barbiturate binding site of the complex and has anxiolytic effects in animals. It is also known to act as an adenosine antagonist at the A1 and A2 subtypes and as a phosphodiesterase inhibitor. Cartazolate was tested in human clinical trials and was found to be efficacious for anxiety but was never marketed. It was developed by a team at E.R. Squibb and Sons in the 1970s.

Bromazolam (XLI-268) is a benzodiazepine derivative which was first synthesised in 1976, but was never marketed. It has subsequently been sold as a designer drug, first being definitively identified by the EMCDDA in Sweden in 2016. It is the bromo instead of chloro analogue of alprazolam, and has similar sedative and anxiolytic effects. Bromazolam is a non subtype selective agonist at the benzodiazepine site of GABAA receptors, with a binding affinity of 2.81nM at the α1 subtype, 0.69nM at α2 and 0.62nM at α5.