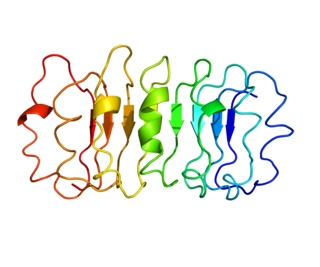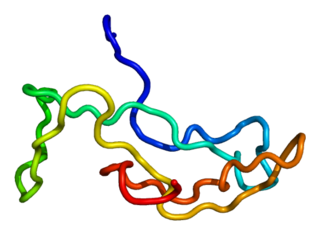
Luteinizing hormone is a hormone produced by gonadotropic cells in the anterior pituitary gland. The production of LH is regulated by gonadotropin-releasing hormone (GnRH) from the hypothalamus. In females, an acute rise of LH known as an LH surge, triggers ovulation and development of the corpus luteum. In males, where LH had also been called interstitial cell–stimulating hormone (ICSH), it stimulates Leydig cell production of testosterone. It acts synergistically with follicle-stimulating hormone (FSH).

Follicle-stimulating hormone (FSH) is a gonadotropin, a glycoprotein polypeptide hormone. FSH is synthesized and secreted by the gonadotropic cells of the anterior pituitary gland and regulates the development, growth, pubertal maturation, and reproductive processes of the body. FSH and luteinizing hormone (LH) work together in the reproductive system.
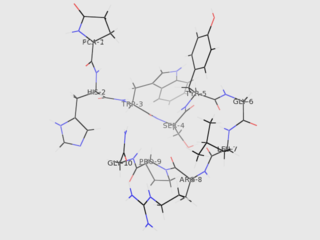
Gonadotropin-releasing hormone (GnRH) is a releasing hormone responsible for the release of follicle-stimulating hormone (FSH) and luteinizing hormone (LH) from the anterior pituitary. GnRH is a tropic peptide hormone synthesized and released from GnRH neurons within the hypothalamus. The peptide belongs to gonadotropin-releasing hormone family. It constitutes the initial step in the hypothalamic–pituitary–gonadal axis.
Gonadotropins are glycoprotein hormones secreted by gonadotropic cells of the anterior pituitary of vertebrates. This family includes the mammalian hormones follicle-stimulating hormone (FSH) and luteinizing hormone (LH), the placental/chorionic gonadotropins, human chorionic gonadotropin (hCG) and equine chorionic gonadotropin (eCG), as well as at least two forms of fish gonadotropins. These hormones are central to the complex endocrine system that regulates normal growth, sexual development, and reproductive function. LH and FSH are secreted by the anterior pituitary gland, while hCG and eCG are secreted by the placenta in pregnant humans and mares, respectively. The gonadotropins act on the gonads, controlling gamete and sex hormone production.

Estrogen receptors (ERs) are a group of proteins found inside cells. They are receptors that are activated by the hormone estrogen (17β-estradiol). Two classes of ER exist: nuclear estrogen receptors, which are members of the nuclear receptor family of intracellular receptors, and membrane estrogen receptors (mERs), which are mostly G protein-coupled receptors. This article refers to the former (ER).
The gonadotropin-releasing hormone receptor (GnRHR), also known as the luteinizing hormone releasing hormone receptor (LHRHR), is a member of the seven-transmembrane, G-protein coupled receptor (GPCR) family. It is the receptor of gonadotropin-releasing hormone (GnRH). The GnRHR is expressed on the surface of pituitary gonadotrope cells as well as lymphocytes, breast, ovary, and prostate.

The follicle-stimulating hormone receptor or FSH receptor (FSHR) is a transmembrane receptor that interacts with the follicle-stimulating hormone (FSH) and represents a G protein-coupled receptor (GPCR). Its activation is necessary for the hormonal functioning of FSH. FSHRs are found in the ovary, testis, and uterus.
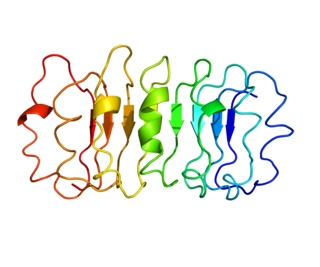
The thyrotropin receptor is a receptor that responds to thyroid-stimulating hormone and stimulates the production of thyroxine (T4) and triiodothyronine (T3). The TSH receptor is a member of the G protein-coupled receptor superfamily of integral membrane proteins and is coupled to the Gs protein.

Kisspeptins are proteins encoded by the KISS1 gene in humans. Kisspeptins are ligands of the G-protein coupled receptor, GPR54. Kiss1 was originally identified as a human metastasis suppressor gene that has the ability to suppress melanoma and breast cancer metastasis. Kisspeptin-GPR54 signaling has an important role in initiating secretion of gonadotropin-releasing hormone (GnRH) at puberty, the extent of which is an area of ongoing research. Gonadotropin-releasing hormone is released from the hypothalamus to act on the anterior pituitary triggering the release of luteinizing hormone (LH), and follicle stimulating hormone (FSH). These gonadotropic hormones lead to sexual maturation and gametogenesis. Disrupting GPR54 signaling can cause hypogonadotrophic hypogonadism in rodents and humans. The Kiss1 gene is located on chromosome 1. It is transcribed in the brain, adrenal gland, and pancreas.

The adrenocorticotropic hormone receptor or ACTH receptor also known as the melanocortin receptor 2 or MC2 receptor is a type of melanocortin receptor (type 2) which is specific for ACTH. A G protein–coupled receptor located on the external cell plasma membrane, it is coupled to Gαs and upregulates levels of cAMP by activating adenylyl cyclase. The ACTH receptor plays a role in immune function and glucose metabolism.

Gonadotropin-releasing hormone receptor is a protein that in humans is encoded by the GNRHR gene.

The KiSS1-derived peptide receptor is a G protein-coupled receptor which binds the peptide hormone kisspeptin (metastin). Kisspeptin is encoded by the metastasis suppressor gene KISS1, which is expressed in a variety of endocrine and gonadal tissues. Activation of the kisspeptin receptor is linked to the phospholipase C and inositol trisphosphate second messenger cascades inside the cell.

Parathyroid hormone/parathyroid hormone-related peptide receptor, also known as parathyroid hormone 1 receptor (PTH1R), is a protein that in humans is encoded by the PTH1R gene. PTH1R functions as a receptor for parathyroid hormone (PTH) and for parathyroid hormone-related protein (PTHrP), also called parathyroid hormone-like hormone (PTHLH).
Progonadoliberin-2 is a protein that in humans is encoded by the GNRH2 gene.

Choriogonadotropin subunit beta variant 1 is a protein that in humans is encoded by the CGB1 gene.

Choriogonadotropin subunit beta variant 2 is a protein that in humans is encoded by the CGB2 gene.

Luteinizing hormone subunit beta also known as lutropin subunit beta or LHβ is a polypeptide that in association with an alpha subunit common to all gonadotropin hormones forms the reproductive signaling molecule luteinizing hormone. In humans it is encoded by the LHB gene.

Choriogonadotropin subunit beta (CG-beta) also known as chorionic gonadotrophin chain beta is a protein that in humans is encoded by the CGB gene.

Leydig cell hypoplasia (LCH), also known as Leydig cell agenesis, is a rare autosomal recessive genetic and endocrine syndrome affecting an estimated 1 in 1,000,000 genetic males. It is characterized by an inability of the body to respond to luteinizing hormone (LH), a gonadotropin which is normally responsible for signaling Leydig cells of the testicles to produce testosterone and other androgen sex hormones. The condition manifests itself as pseudohermaphroditism, hypergonadotropic hypogonadism, reduced or absent puberty, and infertility.
Hypogonadotropic hypogonadism (HH), is due to problems with either the hypothalamus or pituitary gland affecting the hypothalamic-pituitary-gonadal axis. Hypothalamic disorders result from a deficiency in the release of gonadotropic releasing hormone (GnRH), while pituitary gland disorders are due to a deficiency in the release of gonadotropins from the anterior pituitary. GnRH is the central regulator in reproductive function and sexual development via the HPG axis. GnRH is released by GnRH neurons, which are hypothalamic neuroendocrine cells, into the hypophyseal portal system acting on gonadotrophs in the anterior pituitary. The release of gonadotropins, LH and FSH, act on the gonads for the development and maintenance of proper adult reproductive physiology. LH acts on Leydig cells in the male testes and theca cells in the female. FSH acts on Sertoli cells in the male and follicular cells in the female. Combined this causes the secretion of gonadal sex steroids and the initiation of folliculogenesis and spermatogenesis. The production of sex steroids forms a negative feedback loop acting on both the anterior pituitary and hypothalamus causing a pulsatile secretion of GnRH. GnRH neurons lack sex steroid receptors and mediators such as kisspeptin stimulate GnRH neurons for pulsatile secretion of GnRH.