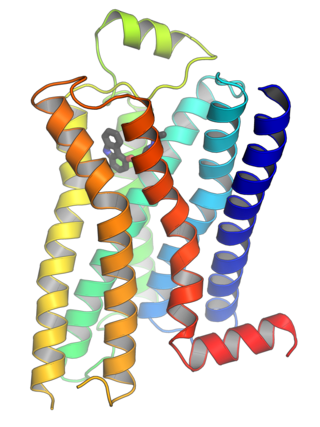
G protein-coupled receptors (GPCRs), also known as seven-(pass)-transmembrane domain receptors, 7TM receptors, heptahelical receptors, serpentine receptors, and G protein-linked receptors (GPLR), form a large group of evolutionarily related proteins that are cell surface receptors that detect molecules outside the cell and activate cellular responses. They are coupled with G proteins. They pass through the cell membrane seven times in the form of six loops of amino acid residues, which is why they are sometimes referred to as seven-transmembrane receptors. Ligands can bind either to the extracellular N-terminus and loops or to the binding site within transmembrane helices. They are all activated by agonists, although a spontaneous auto-activation of an empty receptor has also been observed.

G proteins, also known as guanine nucleotide-binding proteins, are a family of proteins that act as molecular switches inside cells, and are involved in transmitting signals from a variety of stimuli outside a cell to its interior. Their activity is regulated by factors that control their ability to bind to and hydrolyze guanosine triphosphate (GTP) to guanosine diphosphate (GDP). When they are bound to GTP, they are 'on', and, when they are bound to GDP, they are 'off'. G proteins belong to the larger group of enzymes called GTPases.

A hormone is a class of signaling molecules in multicellular organisms that are sent to distant organs or tissues by complex biological processes to regulate physiology and behavior. Hormones are required for the correct development of animals, plants and fungi. Due to the broad definition of a hormone, numerous kinds of molecules can be classified as hormones. Among the substances that can be considered hormones, are eicosanoids, steroids, amino acid derivatives, protein or peptides, and gases.

Signal transduction is the process by which a chemical or physical signal is transmitted through a cell as a series of molecular events. Proteins responsible for detecting stimuli are generally termed receptors, although in some cases the term sensor is used. The changes elicited by ligand binding in a receptor give rise to a biochemical cascade, which is a chain of biochemical events known as a signaling pathway.

A steroid hormone is a steroid that acts as a hormone. Steroid hormones can be grouped into two classes: corticosteroids and sex steroids. Within those two classes are five types according to the receptors to which they bind: glucocorticoids and mineralocorticoids and androgens, estrogens, and progestogens. Vitamin D derivatives are a sixth closely related hormone system with homologous receptors. They have some of the characteristics of true steroids as receptor ligands.

A neurotransmitter receptor is a membrane receptor protein that is activated by a neurotransmitter. Chemicals on the outside of the cell, such as a neurotransmitter, can bump into the cell's membrane, in which there are receptors. If a neurotransmitter bumps into its corresponding receptor, they will bind and can trigger other events to occur inside the cell. Therefore, a membrane receptor is part of the molecular machinery that allows cells to communicate with one another. A neurotransmitter receptor is a class of receptors that specifically binds with neurotransmitters as opposed to other molecules.

In biochemistry and pharmacology, receptors are chemical structures, composed of protein, that receive and transduce signals that may be integrated into biological systems. These signals are typically chemical messengers which bind to a receptor and produce physiological responses such as change in the electrical activity of a cell. For example, GABA, an inhibitory neurotransmitter, inhibits electrical activity of neurons by binding to GABAA receptors. There are three main ways the action of the receptor can be classified: relay of signal, amplification, or integration. Relaying sends the signal onward, amplification increases the effect of a single ligand, and integration allows the signal to be incorporated into another biochemical pathway.
Steroid hormone receptors are found in the nucleus, cytosol, and also on the plasma membrane of target cells. They are generally intracellular receptors and initiate signal transduction for steroid hormones which lead to changes in gene expression over a time period of hours to days. The best studied steroid hormone receptors are members of the nuclear receptor subfamily 3 (NR3) that include receptors for estrogen and 3-ketosteroids. In addition to nuclear receptors, several G protein-coupled receptors and ion channels act as cell surface receptors for certain steroid hormones.
Second messengers are intracellular signaling molecules released by the cell in response to exposure to extracellular signaling molecules—the first messengers. Second messengers trigger physiological changes at cellular level such as proliferation, differentiation, migration, survival, apoptosis and depolarization.
Intracellular receptors are globular protein receptors located inside the cell rather than on its cell membrane. The word intracellular means "within or inside a cell." Molecules that cross a cell membrane to bind with a receptor are generally nonpolar and may be relatively small. These molecules are also known as ligands. Hormones that use intracellular receptors include thyroid, aldosterone, and steroid hormones.
In biology, cell signaling is the process by which a cell interacts with itself, other cells, and the environment. Cell signaling is a fundamental property of all cellular life in prokaryotes and eukaryotes.
The thyroid hormone receptor (TR) is a type of nuclear receptor that is activated by binding thyroid hormone. TRs act as transcription factors, ultimately affecting the regulation of gene transcription and translation. These receptors also have non-genomic effects that lead to second messenger activation, and corresponding cellular response.
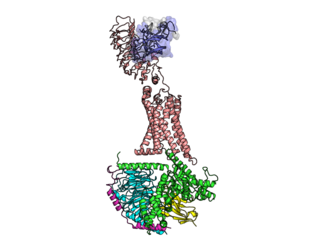
The luteinizing hormone/choriogonadotropin receptor (LHCGR), also lutropin/choriogonadotropin receptor (LCGR) or luteinizing hormone receptor (LHR), is a transmembrane receptor found predominantly in the ovary and testis, but also many extragonadal organs such as the uterus and breasts. The receptor interacts with both luteinizing hormone (LH) and chorionic gonadotropins and represents a G protein-coupled receptor (GPCR). Its activation is necessary for the hormonal functioning during reproduction.
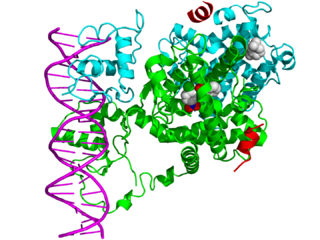
In the field of molecular biology, nuclear receptors are a class of proteins responsible for sensing steroids, thyroid hormones, vitamins, and certain other molecules. These intracellular receptors work with other proteins to regulate the expression of specific genes, thereby controlling the development, homeostasis, and metabolism of the organism.

Guanine nucleotide exchange factors (GEFs) are proteins or protein domains that activate monomeric GTPases by stimulating the release of guanosine diphosphate (GDP) to allow binding of guanosine triphosphate (GTP). A variety of unrelated structural domains have been shown to exhibit guanine nucleotide exchange activity. Some GEFs can activate multiple GTPases while others are specific to a single GTPase.
In the field of molecular biology, the cAMP-dependent pathway, also known as the adenylyl cyclase pathway, is a G protein-coupled receptor-triggered signaling cascade used in cell communication.
Nuclear receptor coregulators are a class of transcription coregulators that have been shown to be involved in any aspect of signaling by any member of the nuclear receptor superfamily. A comprehensive database of coregulators for nuclear receptors and other transcription factors was previously maintained at the Nuclear Receptor Signaling Atlas website which has since been replaced by the Signaling Pathways Project website.

Cell surface receptors are receptors that are embedded in the plasma membrane of cells. They act in cell signaling by receiving extracellular molecules. They are specialized integral membrane proteins that allow communication between the cell and the extracellular space. The extracellular molecules may be hormones, neurotransmitters, cytokines, growth factors, cell adhesion molecules, or nutrients; they react with the receptor to induce changes in the metabolism and activity of a cell. In the process of signal transduction, ligand binding affects a cascading chemical change through the cell membrane.
Membrane progesterone receptors (mPRs) are a group of cell surface receptors and membrane steroid receptors belonging to the progestin and adipoQ receptor (PAQR) family which bind the endogenous progestogen and neurosteroid progesterone, as well as the neurosteroid allopregnanolone. Unlike the progesterone receptor (PR), a nuclear receptor which mediates its effects via genomic mechanisms, mPRs are cell surface receptors which rapidly alter cell signaling via modulation of intracellular signaling cascades. The mPRs mediate important physiological functions in male and female reproductive tracts, liver, neuroendocrine tissues, and the immune system as well as in breast and ovarian cancer.

Membrane estrogen receptors (mERs) are a group of receptors which bind estrogen. Unlike nuclear estrogen receptors, which mediate their effects via slower genomic mechanisms, mERs are cell surface receptors that rapidly alter cell signaling via modulation of intracellular signaling cascades.












