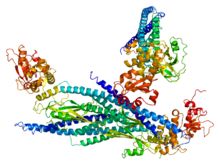
A tyrosine kinase is an enzyme that can transfer a phosphate group from ATP to the tyrosine residues of specific proteins inside a cell. It functions as an "on" or "off" switch in many cellular functions.

The Philadelphia chromosome or Philadelphia translocation (Ph) is a specific genetic abnormality in chromosome 22 of leukemia cancer cells. This chromosome is defective and unusually short because of reciprocal translocation, t(9;22)(q34;q11), of genetic material between chromosome 9 and chromosome 22, and contains a fusion gene called BCR-ABL1. This gene is the ABL1 gene of chromosome 9 juxtaposed onto the breakpoint cluster region BCR gene of chromosome 22, coding for a hybrid protein: a tyrosine kinase signaling protein that is "always on", causing the cell to divide uncontrollably by interrupting the stability of the genome and impairing various signaling pathways governing the cell cycle.
The JAK-STAT signaling pathway is a chain of interactions between proteins in a cell, and is involved in processes such as immunity, cell division, cell death, and tumour formation. The pathway communicates information from chemical signals outside of a cell to the cell nucleus, resulting in the activation of genes through the process of transcription. There are three key parts of JAK-STAT signalling: Janus kinases (JAKs), signal transducer and activator of transcription proteins (STATs), and receptors. Disrupted JAK-STAT signalling may lead to a variety of diseases, such as skin conditions, cancers, and disorders affecting the immune system.

Tyrosine-protein kinase ABL1 also known as ABL1 is a protein that, in humans, is encoded by the ABL1 gene located on chromosome 9. c-Abl is sometimes used to refer to the version of the gene found within the mammalian genome, while v-Abl refers to the viral gene, which was initially isolated from the Abelson murine leukemia virus.
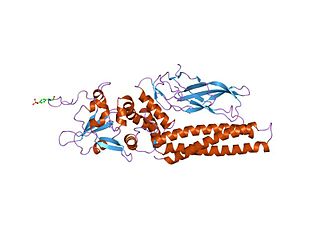
Members of the signal transducer and activator of transcription (STAT) protein family are intracellular transcription factors that mediate many aspects of cellular immunity, proliferation, apoptosis and differentiation. They are primarily activated by membrane receptor-associated Janus kinases (JAK). Dysregulation of this pathway is frequently observed in primary tumors and leads to increased angiogenesis which enhances the survival of tumors and immunosuppression. Gene knockout studies have provided evidence that STAT proteins are involved in the development and function of the immune system and play a role in maintaining immune tolerance and tumor surveillance.
The MAPK/ERK pathway is a chain of proteins in the cell that communicates a signal from a receptor on the surface of the cell to the DNA in the nucleus of the cell.

Receptor tyrosine kinases (RTKs) are the high-affinity cell surface receptors for many polypeptide growth factors, cytokines, and hormones. Of the 90 unique tyrosine kinase genes identified in the human genome, 58 encode receptor tyrosine kinase proteins. Receptor tyrosine kinases have been shown not only to be key regulators of normal cellular processes but also to have a critical role in the development and progression of many types of cancer. Mutations in receptor tyrosine kinases lead to activation of a series of signalling cascades which have numerous effects on protein expression. Receptor tyrosine kinases are part of the larger family of protein tyrosine kinases, encompassing the receptor tyrosine kinase proteins which contain a transmembrane domain, as well as the non-receptor tyrosine kinases which do not possess transmembrane domains.

The erythropoietin receptor (EpoR) is a protein that in humans is encoded by the EPOR gene. EpoR is a 52kDa peptide with a single carbohydrate chain resulting in an approximately 56-57 kDa protein found on the surface of EPO responding cells. It is a member of the cytokine receptor family. EpoR pre-exists as dimers. These dimers were originally thought to be formed by extracellular domain interactions, however, it is now assumed that it is formed by interactions of the transmembrane domain and that the original structure of the extracellular interaction site was due to crystallisation conditions and does not depict the native conformation. Binding of a 30 kDa ligand erythropoietin (Epo), changes the receptor's conformational change, resulting in the autophosphorylation of Jak2 kinases that are pre-associated with the receptor. At present, the most well-established function of EpoR is to promote proliferation and rescue of erythroid progenitors from apoptosis.

Platelet-derived growth factor receptors (PDGF-R) are cell surface tyrosine kinase receptors for members of the platelet-derived growth factor (PDGF) family. PDGF subunits -A and -B are important factors regulating cell proliferation, cellular differentiation, cell growth, development and many diseases including cancer. There are two forms of the PDGF-R, alpha and beta each encoded by a different gene. Depending on which growth factor is bound, PDGF-R homo- or heterodimerizes.

Growth factor receptor-bound protein 2 also known as Grb2 is an adaptor protein involved in signal transduction/cell communication. In humans, the GRB2 protein is encoded by the GRB2 gene.

The breakpoint cluster region protein (BCR) also known as renal carcinoma antigen NY-REN-26 is a protein that in humans is encoded by the BCR gene. BCR is one of the two genes in the BCR-ABL fusion protein, which is associated with the Philadelphia chromosome. Two transcript variants encoding different isoforms have been found for this gene.

Fibroblast growth factor receptor 1 (FGFR1), also known as basic fibroblast growth factor receptor 1, fms-related tyrosine kinase-2 / Pfeiffer syndrome, and CD331, is a receptor tyrosine kinase whose ligands are specific members of the fibroblast growth factor family. FGFR1 has been shown to be associated with Pfeiffer syndrome, and clonal eosinophilias.
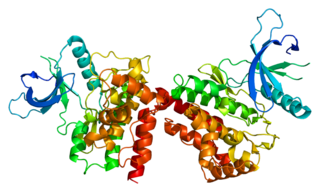
Janus kinase 2 is a non-receptor tyrosine kinase. It is a member of the Janus kinase family and has been implicated in signaling by members of the type II cytokine receptor family, the GM-CSF receptor family, the gp130 receptor family, and the single chain receptors.
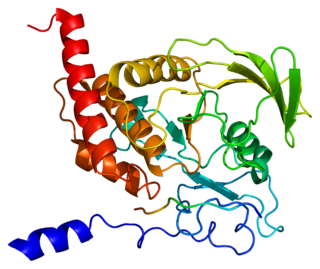
Tyrosine-protein phosphatase non-receptor type 6, also known as Src homology region 2 domain-containing phosphatase-1 (SHP-1), is an enzyme that in humans is encoded by the PTPN6 gene.

Signal transducer and activator of transcription 5A is a protein that in humans is encoded by the STAT5A gene. STAT5A orthologs have been identified in several placentals for which complete genome data are available.

Proto-oncogene tyrosine-protein kinase Src, also known as proto-oncogene c-Src, or simply c-Src, is a non-receptor tyrosine kinase protein that in humans is encoded by the SRC gene. It belongs to a family of Src family kinases and is similar to the v-Src gene of Rous sarcoma virus. It includes an SH2 domain, an SH3 domain and a tyrosine kinase domain. Two transcript variants encoding the same protein have been found for this gene.

Signal transducer and activator of transcription 5B is a protein that in humans is encoded by the STAT5B gene. STAT5B orthologs have been identified in most placentals for which complete genome data are available.
The Akt signaling pathway or PI3K-Akt signaling pathway is a signal transduction pathway that promotes survival and growth in response to extracellular signals. Key proteins involved are PI3K and Akt.
A non-receptor tyrosine kinase (nRTK) is a cytosolic enzyme that is responsible for catalysing the transfer of a phosphate group from a nucleoside triphosphate donor, such as ATP, to tyrosine residues in proteins. Non-receptor tyrosine kinases are a subgroup of protein family tyrosine kinases, enzymes that can transfer the phosphate group from ATP to a tyrosine residue of a protein (phosphorylation). These enzymes regulate many cellular functions by switching on or switching off other enzymes in a cell.

Autophosphorylation is a type of post-translational modification of proteins. It is generally defined as the phosphorylation of the kinase by itself. In eukaryotes, this process occurs by the addition of a phosphate group to serine, threonine or tyrosine residues within protein kinases, normally to regulate the catalytic activity. Autophosphorylation may occur when a kinases' own active site catalyzes the phosphorylation reaction, or when another kinase of the same type provides the active site that carries out the chemistry. The latter often occurs when kinase molecules dimerize. In general, the phosphate groups introduced are gamma phosphates from nucleoside triphosphates, most commonly ATP.