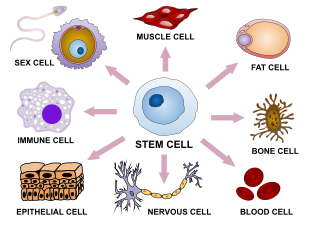
Cellular differentiation is the process in which a cell changes from one cell type to another. Usually, the cell changes to a more specialized type. Differentiation occurs numerous times during the development of a multicellular organism as it changes from a simple zygote to a complex system of tissues and cell types. Differentiation continues in adulthood as adult stem cells divide and create fully differentiated daughter cells during tissue repair and during normal cell turnover. Some differentiation occurs in response to antigen exposure. Differentiation dramatically changes a cell's size, shape, membrane potential, metabolic activity, and responsiveness to signals. These changes are largely due to highly controlled modifications in gene expression and are the study of epigenetics. With a few exceptions, cellular differentiation almost never involves a change in the DNA sequence itself. Thus, different cells can have very different physical characteristics despite having the same genome.

The PAX3 gene encodes a member of the paired box or PAX family of transcription factors. The PAX family consists of nine human (PAX1-PAX9) and nine mouse (Pax1-Pax9) members arranged into four subfamilies. Human PAX3 and mouse Pax3 are present in a subfamily along with the highly homologous human PAX7 and mouse Pax7 genes. The human PAX3 gene is located in the 2q36.1 chromosomal region, and contains 10 exons within a 100 kb region.

Bone morphogenetic protein 4 is a protein that in humans is encoded by BMP4 gene. BMP4 is found on chromosome 14q22-q23

SRY -box 2, also known as SOX2, is a transcription factor that is essential for maintaining self-renewal, or pluripotency, of undifferentiated embryonic stem cells. Sox2 has a critical role in maintenance of embryonic and neural stem cells.
Protein BTG2 also known as BTG family member 2 or NGF-inducible anti-proliferative protein PC3 or NGF-inducible protein TIS21, is a protein that in humans is encoded by the BTG2 gene and in other mammals by the homologous Btg2 gene. This protein controls cell cycle progression and proneural genes expression by acting as a transcription coregulator that enhances or inhibits the activity of transcription factors.

Transcription factor HES1 is a protein that is encoded by the Hes1 gene, and is the mammalian homolog of the hairy gene in Drosophila. HES1 is one of the seven members of the Hes gene family (HES1-7). Hes genes code nuclear proteins that suppress transcription.

Transcription factor Maf also known as proto-oncogene c-Maf or V-maf musculoaponeurotic fibrosarcoma oncogene homolog is a transcription factor that in humans is encoded by the MAF gene.

Gamma-crystallin B is a protein that in humans is encoded by the CRYGB gene.

Hairy/enhancer-of-split related with YRPW motif protein 2 (HEY2) also known as cardiovascular helix-loop-helix factor 1 (CHF1) is a protein that in humans is encoded by the HEY2 gene.

Protein BTG1 is a protein that in humans is encoded by the BTG1 gene.

Protein atonal homolog 1 is a protein that in humans is encoded by the ATOH1 gene.

Transcription factor SOX-11 is a protein that in humans is encoded by the SOX11 gene.
Neurogenins are a family of bHLH transcription factors involved in specifying neuronal differentiation. It is one of many gene families related to the atonal gene in Drosophila. Other positive regulators of neuronal differentiation also expressed during early neural development include NeuroD and ASCL1.

Gamma-crystallin A is a protein that in humans is encoded by the CRYGA gene.

Transcription factor SOX-21 is a protein that in humans is encoded by the SOX21 gene. It is a member of the Sox gene family of transcription factors.

Forkhead box protein A2 (FOXA2), also known as hepatocyte nuclear factor 3-beta (HNF-3B), is a transcription factor that plays an important role during development, in mature tissues and, when dysregulated or mutated, also in cancer.

Eomesodermin also known as T-box brain protein 2 (Tbr2) is a protein that in humans is encoded by the EOMES gene.
Epigenetics is the study of heritable changes in gene expression which do not result from modifications to the sequence of DNA. Neurogenesis is the mechanism for neuron proliferation and differentiation. It entails many different complex processes which are all time and order dependent. Processes such as neuron proliferation, fate specification, differentiation, maturation, and functional integration of newborn cells into existing neuronal networks are all interconnected. In the past decade many epigenetic regulatory mechanisms have been shown to play a large role in the timing and determination of neural stem cell lineages.
Proneural genes encode transcription factors of the basic helix-loop-helix (bHLH) class which are responsible for the development of neuroectodermal progenitor cells. Proneural genes have multiple functions in neural development. They integrate positional information and contribute to the specification of progenitor-cell identity. From the same ectodermal cell types, neural or epidermal cells can develop based on interactions between proneural and neurogenic genes. Neurogenic genes are so called because loss of function mutants show an increase number of developed neural precursors. On the other hand, proneural genes mutants fail to develop neural precursor cells.

Homeobox protein CDX-4 is a protein that in humans is encoded by the CDX4 gene. This gene is a member of the caudal-related homeobox transcription factor family that also includes CDX1 and CDX2.















