Related Research Articles

A muscle cell, also known as a myocyte, is a mature contractile cell in the muscle of an animal. In humans and other vertebrates there are three types: skeletal, smooth, and cardiac (cardiomyocytes). A skeletal muscle cell is long and threadlike with many nuclei and is called a muscle fiber. Muscle cells develop from embryonic precursor cells called myoblasts.

MyoD, also known as myoblast determination protein 1, is a protein in animals that plays a major role in regulating muscle differentiation. MyoD, which was discovered in the laboratory of Harold M. Weintraub, belongs to a family of proteins known as myogenic regulatory factors (MRFs). These bHLH transcription factors act sequentially in myogenic differentiation. Vertebrate MRF family members include MyoD1, Myf5, myogenin, and MRF4 (Myf6). In non-vertebrate animals, a single MyoD protein is typically found.
Myosatellite cells, also known as satellite cells, muscle stem cells or MuSCs, are small multipotent cells with very little cytoplasm found in mature muscle. Satellite cells are precursors to skeletal muscle cells, able to give rise to satellite cells or differentiated skeletal muscle cells. They have the potential to provide additional myonuclei to their parent muscle fiber, or return to a quiescent state. More specifically, upon activation, satellite cells can re-enter the cell cycle to proliferate and differentiate into myoblasts.

A basic helix–loop–helix (bHLH) is a protein structural motif that characterizes one of the largest families of dimerizing transcription factors. The word "basic" does not refer to complexity but to the chemistry of the motif because transcription factors in general contain basic amino acid residues in order to facilitate DNA binding.
Hox genes, a subset of homeobox genes, are a group of related genes that specify regions of the body plan of an embryo along the head-tail axis of animals. Hox proteins encode and specify the characteristics of 'position', ensuring that the correct structures form in the correct places of the body. For example, Hox genes in insects specify which appendages form on a segment, and Hox genes in vertebrates specify the types and shape of vertebrae that will form. In segmented animals, Hox proteins thus confer segmental or positional identity, but do not form the actual segments themselves.

Myogenesis is the formation of skeletal muscular tissue, particularly during embryonic development.

Myogenin, is a transcriptional activator encoded by the MYOG gene. Myogenin is a muscle-specific basic-helix-loop-helix (bHLH) transcription factor involved in the coordination of skeletal muscle development or myogenesis and repair. Myogenin is a member of the MyoD family of transcription factors, which also includes MyoD, Myf5, and MRF4.

Twist-related protein 1 (TWIST1) also known as class A basic helix–loop–helix protein 38 (bHLHa38) is a basic helix-loop-helix transcription factor that in humans is encoded by the TWIST1 gene.
An E-box is a DNA response element found in some eukaryotes that acts as a protein-binding site and has been found to regulate gene expression in neurons, muscles, and other tissues. Its specific DNA sequence, CANNTG, with a palindromic canonical sequence of CACGTG, is recognized and bound by transcription factors to initiate gene transcription. Once the transcription factors bind to the promoters through the E-box, other enzymes can bind to the promoter and facilitate transcription from DNA to mRNA.
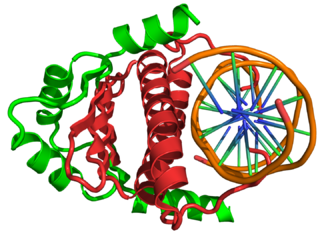
In the field of molecular biology, myocyte enhancer factor-2 (Mef2) proteins are a family of transcription factors which through control of gene expression are important regulators of cellular differentiation and consequently play a critical role in embryonic development. In adult organisms, Mef2 proteins mediate the stress response in some tissues. Mef2 proteins contain both MADS-box and Mef2 DNA-binding domains.

DNA-binding protein inhibitor ID-1 is a protein that in humans is encoded by the ID1 gene.

Myocyte-specific enhancer factor 2A is a protein that in humans is encoded by the MEF2A gene. MEF2A is a transcription factor in the Mef2 family. In humans it is located on chromosome 15q26. Certain mutations in MEF2A cause an autosomal dominant form of coronary artery disease and myocardial infarction.

Transcription factor 12 is a protein that in humans is encoded by the TCF12 gene.

Transcription cofactor HES-6 is a protein that in humans is encoded by the HES6 gene.
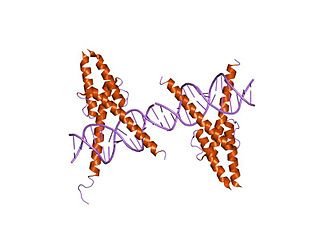
In molecular biology, the myogenic determination factor 5 proteins are a family of proteins found in eukaryotes. This family includes the Myf5 protein, which is responsible for directing cells to the skeletal myocyte lineage during development. Myf5 is likely to act in a similar way to the other MRF4 proteins such as MyoD which perform the same function. These are histone acetyltransferases and histone deacetylases which activate and repress genes involved in the myocyte lineage.

Myogenic factor 5 is a protein that in humans is encoded by the MYF5 gene. It is a protein with a key role in regulating muscle differentiation or myogenesis, specifically the development of skeletal muscle. Myf5 belongs to a family of proteins known as myogenic regulatory factors (MRFs). These basic helix loop helix transcription factors act sequentially in myogenic differentiation. MRF family members include Myf5, MyoD (Myf3), myogenin, and MRF4 (Myf6). This transcription factor is the earliest of all MRFs to be expressed in the embryo, where it is only markedly expressed for a few days. It functions during that time to commit myogenic precursor cells to become skeletal muscle. In fact, its expression in proliferating myoblasts has led to its classification as a determination factor. Furthermore, Myf5 is a master regulator of muscle development, possessing the ability to induce a muscle phenotype upon its forced expression in fibroblastic cells.

Myogenic factor 6 is a protein that in humans is encoded by the MYF6 gene. This gene is also known in the biomedical literature as MRF4 and herculin. MYF6 is a myogenic regulatory factor (MRF) involved in the process known as myogenesis.
Margaret Buckingham, is a British developmental biologist working in the fields of myogenesis and cardiogenesis. She is an honorary professor at the Pasteur Institute in Paris and emeritus director in the Centre national de la recherche scientifique (CNRS). She is a member of the European Molecular Biology Organization, the Academia Europaea and the French Academy of Sciences.
Harold M. "Hal" Weintraub was an American scientist who lived from 1945 until his death in 1995 from an aggressive brain tumor. Only 49 years old, Weintraub left behind a legacy of research.

(HES7) or bHLHb37 is protein coding mammalian gene found on chromosome 17 in humans. HES7 is a member of the Hairy and Enhancer of Split families of Basic helix-loop-helix proteins. The gene product is a transcription factor and is expressed cyclically in the presomitic mesoderm as part of the Notch signalling pathway. HES7 is involved in the segmentation of somites from the presomitic mesoderm in vertebrates. The HES7 gene is self-regulated by a negative feedback loop in which the gene product can bind to its own promoter. This causes the gene to be expressed in an oscillatory manner. The HES7 protein also represses expression of Lunatic Fringe (LFNG) thereby both directly and indirectly regulating the Notch signalling pathway. Mutations in HES7 can result in deformities of the spine, ribs and heart. Spondylocostal dysostosis is a common disease caused by mutations in the HES7 gene. The inheritance pattern of Spondylocostal dysostosis is autosomal recessive.
References
- ↑ Perry R, Rudnick M (2000). "Molecular mechanisms regulating myogenic determination and differentiation". Front Biosci. 5: D750–67. doi: 10.2741/Perry . PMID 10966875.
- ↑ Weintraub H, Davis R, Tapscott S, Thayer M, Krause M, Benezra R, Blackwell T, Turner D, Rupp R, Hollenberg S (1991). "The myoD gene family: nodal point during specification of the muscle cell lineage". Science. 251 (4995): 761–6. Bibcode:1991Sci...251..761W. doi:10.1126/science.1846704. PMID 1846704.
- ↑ Barndt R, Zhuang Y (1999). "Controlling lymphopoiesis with a combinatorial E-protein code". Cold Spring Harb Symp Quant Biol. 64: 45–50. doi:10.1101/sqb.1999.64.45. PMID 11232321.
- ↑ Hernández-Hernández J, García-González E, Brun C, Rudnicki M (2017). "The myogenic regulatory factors, determinants of muscle development, cell identity and regeneration". Seminars in Cell and Developmental Biology. 72: 10–18. doi: 10.1016/j.semcdb.2017.11.010 . PMC 5723221 . PMID 29127045.
- ↑ Aase-Remedios M, Coll-Lladó C, Ferrier D (2020). "More Than One-to-Four via 2R: Evidence of an Independent Amphioxus Expansion and Two-Gene Ancestral Vertebrate State for MyoD-Related Myogenic Regulatory Factors (MRFs)". Molecular Biology and Evolution. doi: 10.1093/molbev/msaa147 . PMC 7530620 . PMID 32520990.