
A muscle cell is also known as a myocyte when referring to either a cardiac muscle cell (cardiomyocyte), or a smooth muscle cell as these are both small cells. A skeletal muscle cell is long and threadlike with many nuclei and is called a muscle fiber. Muscle cells develop from embryonic precursor cells called myoblasts.

MyoD, also known as myoblast determination protein 1, is a protein in animals that plays a major role in regulating muscle differentiation. MyoD, which was discovered in the laboratory of Harold M. Weintraub, belongs to a family of proteins known as myogenic regulatory factors (MRFs). These bHLH transcription factors act sequentially in myogenic differentiation. Vertebrate MRF family members include MyoD1, Myf5, myogenin, and MRF4 (Myf6). In non-vertebrate animals, a single MyoD protein is typically found.
Myosatellite cells, also known as satellite cells, muscle stem cells or MuSCs, are small multipotent cells with very little cytoplasm found in mature muscle. Satellite cells are precursors to skeletal muscle cells, able to give rise to satellite cells or differentiated skeletal muscle cells. They have the potential to provide additional myonuclei to their parent muscle fiber, or return to a quiescent state. More specifically, upon activation, satellite cells can re-enter the cell cycle to proliferate and differentiate into myoblasts.

The PAX3 gene encodes a member of the paired box or PAX family of transcription factors. The PAX family consists of nine human (PAX1-PAX9) and nine mouse (Pax1-Pax9) members arranged into four subfamilies. Human PAX3 and mouse Pax3 are present in a subfamily along with the highly homologous human PAX7 and mouse Pax7 genes. The human PAX3 gene is located in the 2q36.1 chromosomal region, and contains 10 exons within a 100 kb region.

Myogenesis is the formation of skeletal muscular tissue, particularly during embryonic development.

Myogenin, is a transcriptional activator encoded by the MYOG gene. Myogenin is a muscle-specific basic-helix-loop-helix (bHLH) transcription factor involved in the coordination of skeletal muscle development or myogenesis and repair. Myogenin is a member of the MyoD family of transcription factors, which also includes MyoD, Myf5, and MRF4.
Myogenic regulatory factors (MRF) are basic helix-loop-helix (bHLH) transcription factors that regulate myogenesis: MyoD, Myf5, myogenin, and MRF4.
An E-box is a DNA response element found in some eukaryotes that acts as a protein-binding site and has been found to regulate gene expression in neurons, muscles, and other tissues. Its specific DNA sequence, CANNTG, with a palindromic canonical sequence of CACGTG, is recognized and bound by transcription factors to initiate gene transcription. Once the transcription factors bind to the promoters through the E-box, other enzymes can bind to the promoter and facilitate transcription from DNA to mRNA.
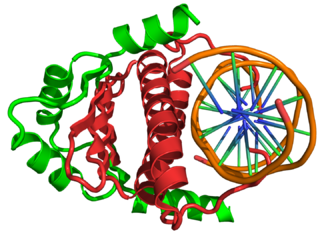
In the field of molecular biology, myocyte enhancer factor-2 (Mef2) proteins are a family of transcription factors which through control of gene expression are important regulators of cellular differentiation and consequently play a critical role in embryonic development. In adult organisms, Mef2 proteins mediate the stress response in some tissues. Mef2 proteins contain both MADS-box and Mef2 DNA-binding domains.

mir-133 is a type of non-coding RNA called a microRNA that was first experimentally characterised in mice. Homologues have since been discovered in several other species including invertebrates such as the fruitfly Drosophila melanogaster. Each species often encodes multiple microRNAs with identical or similar mature sequence. For example, in the human genome there are three known miR-133 genes: miR-133a-1, miR-133a-2 and miR-133b found on chromosomes 18, 20 and 6 respectively. The mature sequence is excised from the 3' arm of the hairpin. miR-133 is expressed in muscle tissue and appears to repress the expression of non-muscle genes.

Muscle tissues are soft tissues that make up the different types of muscles in most animals, and give the ability of muscles to contract. It is also referred to as myopropulsive tissue. Muscle tissue is formed during embryonic development, in a process known as myogenesis. Muscle tissue contains special contractile proteins called actin and myosin which contract and relax to cause movement. Among many other muscle proteins present are two regulatory proteins, troponin and tropomyosin.
Alveolar rhabdomyosarcoma (ARMS) is a subtype of the rhabdomyosarcoma soft tissue cancer family whose lineage is from mesenchymal cells and are related to skeletal muscle cells. ARMS tumors resemble the alveolar tissue in the lungs. Tumor location varies from patient to patient, but is commonly found in the head and neck region, male and female urogenital tracts, the torso, and extremities. Two fusion proteins can be associated with ARMS, but are not necessary, PAX3-FKHR. and PAX7-FKHR. In children and adolescents ARMS accounts for about 1 percent of all malignancies, has an incidence rate of 1 per million, and most cases occur sporadically with no genetic predisposition. PAX3-FOXO1 is now known to drive cancer-promoting gene expression programs through creation of distant genetic elements called super enhancers.

Actin, alpha skeletal muscle is a protein that in humans is encoded by the ACTA1 gene.

Myocyte-specific enhancer factor 2C also known as MADS box transcription enhancer factor 2, polypeptide C is a protein that in humans is encoded by the MEF2C gene. MEF2C is a transcription factor in the Mef2 family.

Myocyte-specific enhancer factor 2A is a protein that in humans is encoded by the MEF2A gene. MEF2A is a transcription factor in the Mef2 family. In humans it is located on chromosome 15q26. Certain mutations in MEF2A cause an autosomal dominant form of coronary artery disease and myocardial infarction.

Paired-like homeodomain transcription factor 2 also known as pituitary homeobox 2 is a protein that in humans is encoded by the PITX2 gene.

Interferon-related developmental regulator 1 is a protein that in humans is encoded by the IFRD1 gene. The gene is expressed mostly in neutrophils, skeletal and cardiac muscle, the brain, and the pancreas. The rat and the mouse homolog genes of interferon-related developmental regulator 1 gene are also known with the name PC4 and Tis21, respectively. IFRD1 is member of a gene family that comprises a second gene, IFRD2, also known as SKmc15.
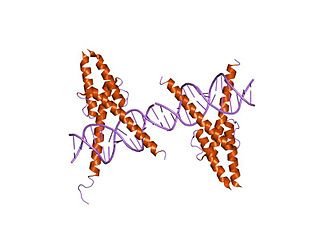
In molecular biology, the myogenic determination factor 5 proteins are a family of proteins found in eukaryotes. This family includes the Myf5 protein, which is responsible for directing cells to the skeletal myocyte lineage during development. Myf5 is likely to act in a similar way to the other MRF4 proteins such as MyoD which perform the same function. These are histone acetyltransferases and histone deacetylases which activate and repress genes involved in the myocyte lineage.

Myogenic factor 6 is a protein that in humans is encoded by the MYF6 gene. This gene is also known in the biomedical literature as MRF4 and herculin. MYF6 is a myogenic regulatory factor (MRF) involved in the process known as myogenesis.
Margaret Buckingham, is a British developmental biologist working in the fields of myogenesis and cardiogenesis. She is an honorary professor at the Pasteur Institute in Paris and emeritus director in the Centre national de la recherche scientifique (CNRS). She is a member of the European Molecular Biology Organization, the Academia Europaea and the French Academy of Sciences.


















