 | |
| Names | |
|---|---|
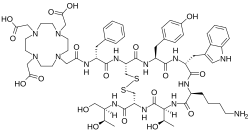
| IUPAC name 2-[4-[2-[[(2R)-1-[[(4R,7S,10S,13R,16S,19R)-10-(4-aminobutyl)-4-[[(2R,3R)-1,3-dihydroxybutan-2-yl]carbamoyl]-7-[(1R)-1-hydroxyethyl]-16-[(4-hydroxyphenyl)methyl]-13-(1H-indol-3-ylmethyl)-6,9,12,15,18-pentaoxo-1,2-dithia-5,8,11,14,17-pentazacycloicos-19-yl]amino]-1-oxo-3-phenylpropan-2-yl]amino]-2-oxoethyl]-7,10-bis(carboxymethyl)-1,4,7,10-tetrazacyclododec-1-yl]acetic acid | |
| Other names SomaKit TOC | |
| Identifiers | |
3D model (JSmol) | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
PubChem CID | |
| UNII | |
CompTox Dashboard (EPA) | |
| |
| |
| Properties | |
| C65H92N14O18S2 | |
| Molar mass | 1421.65 g·mol−1 |
| Pharmacology | |
| License data | |
| Legal status | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Edotreotide (USAN, also known as (DOTA 0-Phe 1-Tyr 3) octreotide, DOTA-TOC, DOTATOC) is a substance which, when bound to various radionuclides, is used in the treatment and diagnosis of certain types of cancer. [3] When used therapeutically it is an example of peptide receptor radionuclide therapy.
