Related Research Articles

The adrenal cortex is the outer region and also the largest part of an adrenal gland. It is divided into three separate zones: zona glomerulosa, zona fasciculata and zona reticularis. Each zone is responsible for producing specific hormones. It is also a secondary site of androgen synthesis.

A steroid hormone is a steroid that acts as a hormone. Steroid hormones can be grouped into two classes: corticosteroids and sex steroids. Within those two classes are five types according to the receptors to which they bind: glucocorticoids and mineralocorticoids and androgens, estrogens, and progestogens. Vitamin D derivatives are a sixth closely related hormone system with homologous receptors. They have some of the characteristics of true steroids as receptor ligands.

Prednisone is a glucocorticoid medication mostly used to suppress the immune system and decrease inflammation in conditions such as asthma, COPD, and rheumatologic diseases. It is also used to treat high blood calcium due to cancer and adrenal insufficiency along with other steroids. It is taken by mouth.

Glucocorticoids are a class of corticosteroids, which are a class of steroid hormones. Glucocorticoids are corticosteroids that bind to the glucocorticoid receptor that is present in almost every vertebrate animal cell. The name "glucocorticoid" is a portmanteau and is composed from its role in regulation of glucose metabolism, synthesis in the adrenal cortex, and its steroidal structure.
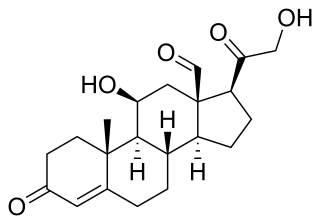
Mineralocorticoids are a class of corticosteroids, which in turn are a class of steroid hormones. Mineralocorticoids are produced in the adrenal cortex and influence salt and water balances. The primary mineralocorticoid is aldosterone.
Corticotropes are basophilic cells in the anterior pituitary that produce pro-opiomelanocortin (POMC) which undergoes cleavage to adrenocorticotropin (ACTH), β-lipotropin (β-LPH), and melanocyte-stimulating hormone (MSH). These cells are stimulated by corticotropin releasing hormone (CRH) and make up 15–20% of the cells in the anterior pituitary. The release of ACTH from the corticotropic cells is controlled by CRH, which is formed in the cell bodies of parvocellular neurosecretory cells within the paraventricular nucleus of the hypothalamus and passes to the corticotropes in the anterior pituitary via the hypophyseal portal system. Adrenocorticotropin hormone stimulates the adrenal cortex to release glucocorticoids and plays an important role in the stress response.
Steroid hormone receptors are found in the nucleus, cytosol, and also on the plasma membrane of target cells. They are generally intracellular receptors and initiate signal transduction for steroid hormones which lead to changes in gene expression over a time period of hours to days. The best studied steroid hormone receptors are members of the nuclear receptor subfamily 3 (NR3) that include receptors for estrogen and 3-ketosteroids. In addition to nuclear receptors, several G protein-coupled receptors and ion channels act as cell surface receptors for certain steroid hormones.
Methylprednisolone is a synthetic glucocorticoid, primarily prescribed for its anti-inflammatory and immunosuppressive effects. It is either used at low doses for chronic illnesses or used concomitantly at high doses during acute flares. Methylprednisolone and its derivatives can be administered orally or parenterally.

The glucocorticoid receptor also known as NR3C1 is the receptor to which cortisol and other glucocorticoids bind.

Corticosteroid 11-β-dehydrogenase isozyme 2 also known as 11-β-hydroxysteroid dehydrogenase 2 is an enzyme that in humans is encoded by the HSD11B2 gene.

The vitamin D receptor (VDR also known as the calcitriol receptor) is a member of the nuclear receptor family of transcription factors. Calcitriol (the active form of vitamin D, 1,25-(OH)2vitamin D3) binds to VDR, which then forms a heterodimer with the retinoid-X receptor. The VDR heterodimer then enters the nucleus and binds to Vitamin D responsive elements (VDRE) in genomic DNA. VDR binding results in expression or transrepression of many specific gene products. VDR is also involved in microRNA-directed post transcriptional mechanisms. In humans, the vitamin D receptor is encoded by the VDR gene located on chromosome 12q13.11.
In the field of molecular biology, nuclear receptors are a class of proteins found within cells that are responsible for sensing steroid and thyroid hormones and certain other molecules. In response, these receptors work with other proteins to regulate the expression of specific genes, thereby controlling the development, homeostasis, and metabolism of the organism.
The nuclear receptor coactivator 2 also known as NCoA-2 is a protein that in humans is encoded by the NCOA2 gene. NCoA-2 is also frequently called glucocorticoid receptor-interacting protein 1 (GRIP1), steroid receptor coactivator-2 (SRC-2), or transcriptional mediators/intermediary factor 2 (TIF2).

G protein-coupled estrogen receptor 1 (GPER), also known as G protein-coupled receptor 30 (GPR30), is a protein that in humans is encoded by the GPER gene. GPER binds to and is activated by the female sex hormone estradiol and is responsible for some of the rapid effects that estradiol has on cells.

Selective glucocorticoid receptor modulators (SEGRMs) and selective glucocorticoid receptor agonists (SEGRAs) formerly known as dissociated glucocorticoid receptor agonists (DIGRAs) are a class of experimental drugs designed to share many of the desirable anti-inflammatory, immunosuppressive, or anticancer properties of classical glucocorticoid drugs but with fewer side effects such as skin atrophy. Although preclinical evidence on SEGRAMs’ anti-inflammatory effects are culminating, currently, the efficacy of these SEGRAMs on cancer are largely unknown.
Membrane progesterone receptors (mPRs) are a group of cell surface receptors and membrane steroid receptors belonging to the progestin and adipoQ receptor (PAQR) family which bind the endogenous progestogen and neurosteroid progesterone, as well as the neurosteroid allopregnanolone. Unlike the progesterone receptor (PR), a nuclear receptor which mediates its effects via genomic mechanisms, mPRs are cell surface receptors which rapidly alter cell signaling via modulation of intracellular signaling cascades. The mPRs mediate important physiological functions in male and female reproductive tracts, liver, neuroendocrine tissues, and the immune system as well as in breast and ovarian cancer.

The corticosteroid receptors are receptors for corticosteroids. They include the following two nuclear receptors:
Membrane androgen receptors (mARs) are a group of G protein-coupled receptors (GPCRs) which bind and are activated by testosterone and/or other androgens. Unlike the androgen receptor (AR), a nuclear receptor which mediates its effects via genomic mechanisms, mARs are cell surface receptors which rapidly alter cell signaling via modulation of intracellular signaling cascades. Known or proposed mARs include ZIP9 and GPRC6A.
Membrane steroid receptors (mSRs), also called extranuclear steroid receptors, are a class of cell surface receptors activated by endogenous steroids that mediate rapid, non-genomic signaling via modulation of intracellular signaling cascades. mSRs are another means besides classical nuclear steroid hormone receptors (SHRs) for steroids to mediate their biological effects. SHRs can produce slow genomic responses or rapid, non-genomic responses in the case of mSRs.
Membrane mineralocorticoid receptors (mMRs) or membrane aldosterone receptors are a group of receptors which bind and are activated by mineralocorticoids such as aldosterone. Unlike the classical nuclear mineralocorticoid receptor (MR), which mediates its effects via genomic mechanisms, mMRs are cell surface receptors which rapidly alter cell signaling via modulation of intracellular signaling cascades. The identities of the mMRs have yet to be fully elucidated, but are thought to include membrane-associated classical MRs as well as yet-to-be-characterized G protein-coupled receptors (GPCRs). Rapid effects of aldosterone were found not be reversed by the MR antagonist spironolactone, indicating additional receptors besides just the classical MR. It has been estimated that as much as 50% of the rapid actions of aldosterone are mediated by mMRs that are not the classical MR, based on findings of insensitivity to classical mR antagonists.
References
- 1 2 Tasker JG, Di S, Malcher-Lopes R (2006). "Minireview: rapid glucocorticoid signaling via membrane-associated receptors". Endocrinology. 147 (12): 5549–56. doi:10.1210/en.2006-0981. PMC 3280589 . PMID 16946006.
- 1 2 Stahn C, Buttgereit F (2008). "Genomic and nongenomic effects of glucocorticoids". Nat Clin Pract Rheumatol. 4 (10): 525–33. doi:10.1038/ncprheum0898. PMID 18762788.
- 1 2 3 Grzanka A, Jarzab J (2009). "[Nongenomic effects of glucocorticoids]". Pneumonol Alergol Pol (in Polish). 77 (4): 387–93. PMID 19722144.
- 1 2 3 Di S, Tasker JG (2008). "Rapid synapse-specific regulation of hypothalamic magnocellular neurons by glucocorticoids". Advances in Vasopressin and Oxytocin — from Genes to Behaviour to Disease. Prog. Brain Res. Progress in Brain Research. Vol. 170. pp. 379–88. doi:10.1016/S0079-6123(08)00431-7. ISBN 9780444532015. PMID 18655897.
- 1 2 Löwenberg M, Stahn C, Hommes DW, Buttgereit F (2008). "Novel insights into mechanisms of glucocorticoid action and the development of new glucocorticoid receptor ligands". Steroids. 73 (9–10): 1025–9. doi:10.1016/j.steroids.2007.12.002. PMID 18221974.
- ↑ Wang C, Liu Y, Cao JM (2014). "G protein-coupled receptors: extranuclear mediators for the non-genomic actions of steroids". Int J Mol Sci. 15 (9): 15412–25. doi: 10.3390/ijms150915412 . PMC 4200746 . PMID 25257522.
- 1 2 3 Groeneweg FL, Karst H, de Kloet ER, Joëls M (2011). "Rapid non-genomic effects of corticosteroids and their role in the central stress response". J. Endocrinol. 209 (2): 153–67. doi: 10.1530/JOE-10-0472 . PMID 21357682.
- ↑ Song IH, Buttgereit F (2006). "Non-genomic glucocorticoid effects to provide the basis for new drug developments". Mol. Cell. Endocrinol. 246 (1–2): 142–6. doi:10.1016/j.mce.2005.11.012. PMID 16388891.
- 1 2 Kawata M, Hirahara-Wada Y, Matsuda K (2008). "[Membrane-associated steroid hormone receptors: functional significance of nongenomic action]". Nippon Rinsho (in Japanese). 66 (1): 55–62. PMID 18193544.
- ↑ Stellato C (2004). "Post-transcriptional and nongenomic effects of glucocorticoids". Proc Am Thorac Soc. 1 (3): 255–63. doi:10.1513/pats.200402-015MS. PMID 16113443.
- ↑ Urbach V, Verriere V, Grumbach Y, Bousquet J, Harvey BJ (2006). "Rapid anti-secretory effects of glucocorticoids in human airway epithelium". Steroids. 71 (4): 323–8. doi:10.1016/j.steroids.2005.09.014. PMID 16298406.
- ↑ Sarabdjitsingh RA, Joëls M, de Kloet ER (2012). "Glucocorticoid pulsatility and rapid corticosteroid actions in the central stress response". Physiol. Behav. 106 (1): 73–80. doi:10.1016/j.physbeh.2011.09.017. PMID 21971364.
- ↑ Groeneweg FL, Karst H, de Kloet ER, Joëls M (2012). "Mineralocorticoid and glucocorticoid receptors at the neuronal membrane, regulators of nongenomic corticosteroid signalling". Mol. Cell. Endocrinol. 350 (2): 299–309. doi:10.1016/j.mce.2011.06.020. PMID 21736918.
- ↑ Löwenberg M, Verhaar AP, van den Brink GR, Hommes DW (2007). "Glucocorticoid signaling: a nongenomic mechanism for T-cell immunosuppression". Trends Mol Med. 13 (4): 158–63. doi:10.1016/j.molmed.2007.02.001. PMID 17293163.
- ↑ Samarasinghe RA, Witchell SF, DeFranco DB (2012). "Cooperativity and complementarity: synergies in non-classical and classical glucocorticoid signaling". Cell Cycle. 11 (15): 2819–27. doi:10.4161/cc.21018. PMC 3419059 . PMID 22801547.