
Aldosterone is the main mineralocorticoid steroid hormone produced by the zona glomerulosa of the adrenal cortex in the adrenal gland. It is essential for sodium conservation in the kidney, salivary glands, sweat glands, and colon. It plays a central role in the homeostatic regulation of blood pressure, plasma sodium (Na+), and potassium (K+) levels. It does so primarily by acting on the mineralocorticoid receptors in the distal tubules and collecting ducts of the nephron. It influences the reabsorption of sodium and excretion of potassium (from and into the tubular fluids, respectively) of the kidney, thereby indirectly influencing water retention or loss, blood pressure, and blood volume. When dysregulated, aldosterone is pathogenic and contributes to the development and progression of cardiovascular and kidney disease. Aldosterone has exactly the opposite function of the atrial natriuretic hormone secreted by the heart.

Mineralocorticoids are a class of corticosteroids, which in turn are a class of steroid hormones. Mineralocorticoids are produced in the adrenal cortex and influence salt and water balances. The primary mineralocorticoid is aldosterone.
Spironolactone, sold under the brand name Aldactone among others, is a medication that is primarily used to treat fluid build-up due to heart failure, liver scarring, or kidney disease. It is also used in the treatment of high blood pressure, low blood potassium that does not improve with supplementation, early puberty in boys, acne and excessive hair growth in women, and as a part of feminizing hormone therapy in trans women. Spironolactone is taken by mouth.
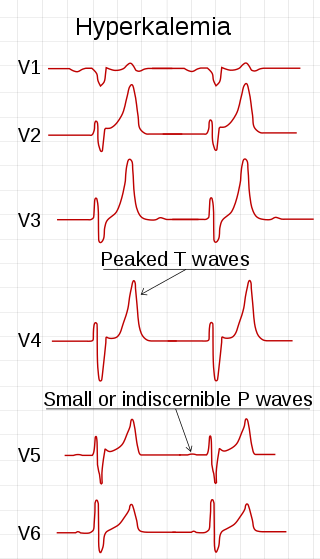
Hyperkalemia is an elevated level of potassium (K+) in the blood. Normal potassium levels are between 3.5 and 5.0 mmol/L (3.5 and 5.0 mEq/L) with levels above 5.5 mmol/L defined as hyperkalemia. Typically hyperkalemia does not cause symptoms. Occasionally when severe it can cause palpitations, muscle pain, muscle weakness, or numbness. Hyperkalemia can cause an abnormal heart rhythm which can result in cardiac arrest and death.

Amiloride, sold under the trade name Midamor among others, is a medication typically used with other medications to treat high blood pressure or swelling due to heart failure or cirrhosis of the liver. Amiloride is classified as a potassium-sparing diuretic. Amiloride is often used together with another diuretic, such as a thiazide or loop diuretic. It is taken by mouth. Onset of action is about two hours and it lasts for about a day.

Potassium-sparing diuretics refers to drugs that cause diuresis without causing potassium loss in the urine. They are typically used as an adjunct in management of hypertension, cirrhosis, and congestive heart failure. The steroidal aldosterone antagonists can also be used for treatment of primary hyperaldosteronism. Spironolactone, a steroidal aldosterone antagonist, is also used in management of female hirsutism and acne from PCOS or other causes.

Hyperaldosteronism is a medical condition wherein too much aldosterone is produced by the adrenal glands, which can lead to lowered levels of potassium in the blood (hypokalemia) and increased hydrogen ion excretion (alkalosis).

Eplerenone, sold under the brand name Inspra, is an aldosterone antagonist type of potassium-sparing diuretic that is used to treat chronic heart failure and high blood pressure, particularly for patients with resistant hypertension due to elevated aldosterone. It is a steroidal antimineralocorticoid of the spirolactone group and a selective aldosterone receptor antagonist (SARA). Eplerenone is more selective than spironolactone at the mineralocorticoid receptor relative to binding at androgen, progestogen, glucocorticoid, or estrogen receptors.

Bartter syndrome (BS) is a rare inherited disease characterised by a defect in the thick ascending limb of the loop of Henle, which results in low potassium levels (hypokalemia), increased blood pH (alkalosis), and normal to low blood pressure. There are two types of Bartter syndrome: neonatal and classic. A closely associated disorder, Gitelman syndrome, is milder than both subtypes of Bartter syndrome.

Pseudohypoaldosteronism (PHA) is a condition that mimics hypoaldosteronism. However, the condition is due to a failure of response to aldosterone, and levels of aldosterone are actually elevated, due to a lack of feedback inhibition.

The mineralocorticoid receptor, also known as the aldosterone receptor or nuclear receptor subfamily 3, group C, member 2, (NR3C2) is a protein that in humans is encoded by the NR3C2 gene that is located on chromosome 4q31.1-31.2.

Canrenone, sold under the brand names Contaren, Luvion, Phanurane, and Spiroletan, is a steroidal antimineralocorticoid of the spirolactone group related to spironolactone which is used as a diuretic in Europe, including in Italy and Belgium. It is also an important active metabolite of spironolactone, and partially accounts for its therapeutic effects.
In physiology, aldosterone escape is a term that has been used to refer to two distinct phenomena involving aldosterone that are exactly opposite each other:
- Escape from the sodium-retaining effects of excess aldosterone in primary hyperaldosteronism, manifested by volume and/or pressure natriuresis.
- The inability of ACE inhibitor therapy to reliably suppress aldosterone release, for example, in patients with heart failure or diabetes, usually manifested by increased salt and water retention. This latter sense may rather be termed refractory hyperaldosteronism.

Spirolactones are a class of functional group in organic chemistry featuring a cyclic ester attached spiro to another ring system. The name is also used to refer to a class of synthetic steroids, called steroid-17α-spirolactones, 17α-spirolactosteroids, or simply 17α-spirolactones, which feature their spirolactone group at the C17α position. They are antimineralocorticoids, or antagonists of the mineralocorticoid receptor, and have been employed clinically as potassium-sparing diuretics. Some also possess progestogenic and/or antiandrogen properties, which have both contributed to side effects and been utilized for medical indications. The spirolactones were developed by G. D. Searle & Company in the 1950s and thereafter and were denoted as "SC" compounds.

A diuretic is any substance that promotes diuresis, the increased production of urine. This includes forced diuresis. A diuretic tablet is sometimes colloquially called a water tablet. There are several categories of diuretics. All diuretics increase the excretion of water from the body, through the kidneys. There exist several classes of diuretic, and each works in a distinct way. Alternatively, an antidiuretic, such as vasopressin, is an agent or drug which reduces the excretion of water in urine.

Mexrenone is a steroidal antimineralocorticoid of the spirolactone group related to spironolactone that was never marketed. It is the lactonic form of mexrenoic acid (mexrenoate), and mexrenoate potassium (SC-26714), the potassium salt of mexrenoic acid, also exists. In addition to the mineralocorticoid receptor, mexrenone also binds to the glucocorticoid, androgen, and progesterone receptors. Relative to spironolactone, it has markedly reduced antiandrogen activity. Eplerenone is the 9-11α-epoxy analogue of mexrenone.

Finerenone, sold under the brand name Kerendia, is a medication used to reduce the risk of kidney function decline, kidney failure, cardiovascular death, non-fatal heart attacks, and hospitalization for heart failure in adults with chronic kidney disease associated with type 2 diabetes. Finerenone is a non-steroidal mineralocorticoid receptor antagonist (MRA). It is taken orally.

Esaxerenone (INN) is a nonsteroidal antimineralocorticoid which was discovered by Exelixis and developed by Daiichi Sankyo Company and is approved in Japan for the treatment of hypertension. It acts as a highly selective silent antagonist of the mineralocorticoid receptor (MR), the receptor for aldosterone, with greater than 1,000-fold selectivity for this receptor over other steroid hormone receptors, and 4-fold and 76-fold higher affinity for the MR relative to the existing antimineralocorticoids spironolactone and eplerenone. As of January 2019, esaxerenone is in phase III clinical trials for diabetic nephropathies.

The pharmacodynamics of spironolactone, an antimineralocorticoid and antiandrogen medication, concern its mechanisms of action, including its biological targets and activities, as well as its physiological effects. The pharmacodynamics of spironolactone are characterized by high antimineralocorticoid activity, moderate antiandrogenic activity, and weak steroidogenesis inhibition. In addition, spironolactone has sometimes been found to increase estradiol and cortisol levels and hence could have slight indirect estrogenic and glucocorticoid effects. The medication has also been found to interact very weakly with the estrogen and progesterone receptors, and to act as an agonist of the pregnane X receptor. Likely due to increased activation of the estrogen and/or progesterone receptors, spironolactone has very weak but significant antigonadotropic effects.























