ATC code J04Antimycobacterials is a therapeutic subgroup of the Anatomical Therapeutic Chemical Classification System, a system of alphanumeric codes developed by the World Health Organization (WHO) for the classification of drugs and other medical products. Subgroup J04 is part of the anatomical group J Antiinfectives for systemic use.

The Einhorn–Brunner reaction is the designation for the chemical reaction of imides with alkyl hydrazines to form an isomeric mixture of 1,2,4-triazoles. It was initially described by the German chemist Alfred Einhorn in a paper, published in 1905, describing N-methylol compounds of amides. In 1914 chemist Karl Brunner published a paper expanding on Einhorn's research of the reaction pictured below, thus resulting in the naming as the Einhorn-Brunner. Substituted 1,2,4-triazole have been prepared from diverse imides and hydrazines.

Ambroxol is a drug that breaks up phlegm, used in the treatment of respiratory diseases associated with viscid or excessive mucus. Ambroxol is often administered as an active ingredient in cough syrup.

The interferon-gamma receptor (IFNGR) protein complex is the heterodimer of two chains: IFNGR1 and IFNGR2. It binds interferon-γ, the sole member of interferon type II.
Mycobacterium florentinum is a strain of bacteria found in humans that can cause infections and other disease conditions, and prolong sickness. It presents a high resistance to antimycobacterial drugs. It is characterized by: slow growth and a short helix 18 in the 16S rDNA.
Mycobacterium heckeshornense is a species of the phylum Actinomycetota, belonging to the genus Mycobacterium.

Copaifera is a genus of tropical plants in the legume family Fabaceae.

Thiocarlide is a thiourea drug used in the treatment of tuberculosis, inhibiting synthesis of oleic acid and tuberculostearic acid.
The Pellizzari reaction was discovered in 1911 by Guido Pellizzari, and is the organic reaction of an amide and a hydrazide to form a 1,2,4-triazole.
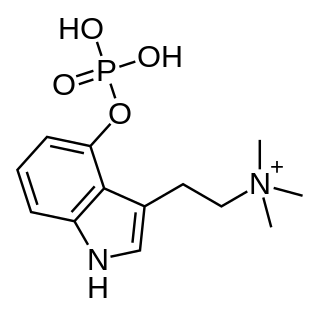
Aeruginascin or N,N,N-trimethyl-4-phosphoryloxytryptamine is an indoleamine derivative which occurs naturally within the mushrooms Inocybe aeruginascens and Pholiotina cyanopus, and Psilocybe cubensis. Aeruginascin is the N-trimethyl analogue of psilocybin. It is closely related to the frog skin toxin bufotenidine (5-HTQ), a potent 5-HT3 receptor agonist, but the aeruginascin metabolite 4-HO-TMT shows strong binding at the 5-HT2 receptors similar to psilocin. The first scientific literature about the pharmacological effects of aeruginascin is from a study published by Gartz in 1989. Across 23 analyzed cases of accidental hallucinogenic mushroom poisonings, people who had ingested the mushroom Inocybe aeruginascens reported only euphoric experiences. This is in contrast to the slight and in some cases extremely dysphoric experiences reported from the accidental ingestion of non aeruginascin containing mushrooms (containing solely psilocybin and psilocin).

An arabinosyltransferase is a transferase enzyme acting upon arabinose. This enzyme is involved in polymerisation of arabinogalactan. Mycobacterially, the more precise term is arabinofuranosyltransferase, since the arabinose residues occur only in a furanose form.
ATCvet code QJ54Antimycobacterials for intramammary use is a therapeutic subgroup of the Anatomical Therapeutic Chemical Classification System for veterinary medicinal products, a system of alphanumeric codes developed by the World Health Organization (WHO) for the classification of drugs and other medical products for veterinary use. Subgroup QJ54 is part of the anatomical group QJ Antiinfectives for systemic use.
The Infectious Disease Pharmacokinetics Laboratory (IDPL) is a research facility that is affiliated with the College of Pharmacy at the University of Florida.
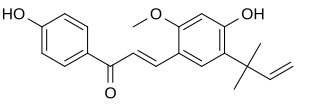
Licochalcone A is a chalconoid, a type of natural phenol. It can be isolated from the root of Glycyrrhiza glabra (liquorice) or Glycyrrhiza inflata. It shows antimalarial, anticancer, antibacterial and antiviral properties in vitro.

Rhus taitensis is a small tree or shrub in the sumac family of plants. It is found from tropical Asia, to Australia and many islands of the Pacific ocean. The chemical tetrahydroxysqualene from dried and ground parts of R. taitensis has in vitro activity against Mycobacterium tuberculosis and the plant has been used in folk medicine locally to treat diarrhea and hearing loss.
Geranylgeraniol is a diterpenoid alcohol. It is a colorless waxy solid.

Chlorophytum inornatum is a flowering plant species in the genus Chlorophytum. It is the type species of its genus. It is related to the commonly known plant Chlorophytum also referred to as a "spider plant". 3-(4'-Methoxybenzyl)-7,8-methylenedioxy-chroman-4-one, a homoisoflavanone with antimycobacterial activity, can be isolated from C. inornatum.
Sunrise University is an Indian private university located in Alwar, Rajasthan. Spread over 30 acre campus, the university was established under Sunrise University Act, 2011 by Government of Rajasthan, and is recognised by UGC.

Salazinic acid is a depsidone with a lactone ring. It is found in some lichens, and is especially prevalent in Parmotrema and Bulbothrix, where its presence or absence is often used to help classify species in those genera.

Lichexanthone is an organic compound in the structural class of chemicals known as xanthones. Lichexanthone was first isolated and identified by Japanese chemists from a species of leafy lichen in the 1940s. The compound is known to occur in many lichens, and it is important in the taxonomy of species in several genera, such as Pertusaria and Pyxine. More than a dozen lichen species have a variation of the word lichexanthone incorporated as part of their binomial name. The presence of lichexanthone in lichens causes them to fluoresce a greenish-yellow colour under long-wavelength UV light; this feature is used to help identify some species. Lichexanthone is also found in several plants, and some species of fungi that do not form lichens.











