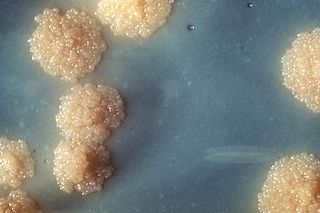
Mycobacterium tuberculosis, also known as Koch's bacillus, is a species of pathogenic bacteria in the family Mycobacteriaceae and the causative agent of tuberculosis. First discovered in 1882 by Robert Koch, M. tuberculosis has an unusual, waxy coating on its cell surface primarily due to the presence of mycolic acid. This coating makes the cells impervious to Gram staining, and as a result, M. tuberculosis can appear weakly Gram-positive. Acid-fast stains such as Ziehl–Neelsen, or fluorescent stains such as auramine are used instead to identify M. tuberculosis with a microscope. The physiology of M. tuberculosis is highly aerobic and requires high levels of oxygen. Primarily a pathogen of the mammalian respiratory system, it infects the lungs. The most frequently used diagnostic methods for tuberculosis are the tuberculin skin test, acid-fast stain, culture, and polymerase chain reaction.

Mycobacterium is a genus of over 190 species in the phylum Actinomycetota, assigned its own family, Mycobacteriaceae. This genus includes pathogens known to cause serious diseases in mammals, including tuberculosis and leprosy in humans. The Greek prefix myco- means 'fungus', alluding to this genus' mold-like colony surfaces. Since this genus has cell walls with Gram-positive and Gram-negative features, acid-fast staining is used to emphasize their resistance to acids, compared to other cell types.
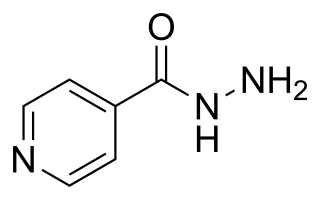
Isoniazid, also known as isonicotinic acid hydrazide (INH), is an antibiotic used for the treatment of tuberculosis. For active tuberculosis it is often used together with rifampicin, pyrazinamide, and either streptomycin or ethambutol. For latent tuberculosis it is often used by itself. It may also be used for atypical types of mycobacteria, such as M. avium, M. kansasii, and M. xenopi. It is usually taken by mouth but may be used by injection into muscle.

Prenylation is the addition of hydrophobic molecules to a protein or a biomolecule. It is usually assumed that prenyl groups (3-methylbut-2-en-1-yl) facilitate attachment to cell membranes, similar to lipid anchors like the GPI anchor, though direct evidence of this has not been observed. Prenyl groups have been shown to be important for protein–protein binding through specialized prenyl-binding domains.
DD-transpeptidase is a bacterial enzyme that catalyzes the transfer of the R-L-aca-D-alanyl moiety of R-L-aca-D-alanyl-D-alanine carbonyl donors to the γ-OH of their active-site serine and from this to a final acceptor. It is involved in bacterial cell wall biosynthesis, namely, the transpeptidation that crosslinks the peptide side chains of peptidoglycan strands.

Glutathione S-transferases (GSTs), previously known as ligandins, are a family of eukaryotic and prokaryotic phase II metabolic isozymes best known for their ability to catalyze the conjugation of the reduced form of glutathione (GSH) to xenobiotic substrates for the purpose of detoxification. The GST family consists of three superfamilies: the cytosolic, mitochondrial, and microsomal—also known as MAPEG—proteins. Members of the GST superfamily are extremely diverse in amino acid sequence, and a large fraction of the sequences deposited in public databases are of unknown function. The Enzyme Function Initiative (EFI) is using GSTs as a model superfamily to identify new GST functions.

Ethambutol is a medication primarily used to treat tuberculosis. It is usually given in combination with other tuberculosis medications, such as isoniazid, rifampicin and pyrazinamide. It may also be used to treat Mycobacterium avium complex, and Mycobacterium kansasii. It is taken by mouth.

Terminal deoxynucleotidyl transferase (TdT), also known as DNA nucleotidylexotransferase (DNTT) or terminal transferase, is a specialized DNA polymerase expressed in immature, pre-B, pre-T lymphoid cells, and acute lymphoblastic leukemia/lymphoma cells. TdT adds N-nucleotides to the V, D, and J exons of the TCR and BCR genes during antibody gene recombination, enabling the phenomenon of junctional diversity. In humans, terminal transferase is encoded by the DNTT gene. As a member of the X family of DNA polymerase enzymes, it works in conjunction with polymerase λ and polymerase μ, both of which belong to the same X family of polymerase enzymes. The diversity introduced by TdT has played an important role in the evolution of the vertebrate immune system, significantly increasing the variety of antigen receptors that a cell is equipped with to fight pathogens. Studies using TdT knockout mice have found drastic reductions (10-fold) in T-cell receptor (TCR) diversity compared with that of normal, or wild-type, systems. The greater diversity of TCRs that an organism is equipped with leads to greater resistance to infection. Although TdT was one of the first DNA polymerases identified in mammals in 1960, it remains one of the least understood of all DNA polymerases. In 2016–18, TdT was discovered to demonstrate in trans template dependant behaviour in addition to its more broadly known template independent behaviour

Glycosyltransferases are enzymes that establish natural glycosidic linkages. They catalyze the transfer of saccharide moieties from an activated nucleotide sugar to a nucleophilic glycosyl acceptor molecule, the nucleophile of which can be oxygen- carbon-, nitrogen-, or sulfur-based.

Nuclear factor erythroid 2-related factor 2 (NRF2), also known as nuclear factor erythroid-derived 2-like 2, is a transcription factor that in humans is encoded by the NFE2L2 gene. NRF2 is a basic leucine zipper (bZIP) protein that may regulate the expression of antioxidant proteins that protect against oxidative damage triggered by injury and inflammation, according to preliminary research. In vitro, NRF2 binds to antioxidant response elements (AREs) in the promoter regions of genes encoding cytoprotective proteins. NRF2 induces the expression of heme oxygenase 1 in vitro leading to an increase in phase II enzymes. NRF2 also inhibits the NLRP3 inflammasome.

Glutathione S-transferase A1 is an enzyme that in humans is encoded by the GSTA1 gene.
In enzymology, a geranyltranstransferase is an enzyme that catalyzes the chemical reaction
In enzymology, an indolylacetylinositol arabinosyltransferase is an enzyme that catalyzes the chemical reaction

Glutathione S-transferase M3 (brain), also known as GSTM2, is an enzyme which in humans is encoded by the GSTM99
Glutathione S-transferase Mu 4 is an enzyme that in humans is encoded by the GSTM4 gene.

Cord factor, or trehalose dimycolate, is a glycolipid molecule found in the cell wall of Mycobacterium tuberculosis and similar species. It is the primary lipid found on the exterior of M. tuberculosis cells. Cord factor influences the arrangement of M. tuberculosis cells into long and slender formations, giving its name. Cord factor is virulent towards mammalian cells and critical for survival of M. tuberculosis in hosts, but not outside of hosts. Cord factor has been observed to influence immune responses, induce the formation of granulomas, and inhibit tumor growth. The antimycobacterial drug SQ109 is thought to inhibit TDM production levels and in this way disrupts its cell wall assembly.
Rhamnopyranosyl-N-acetylglucosaminyl-diphospho-decaprenol beta-1,3/1,4-galactofuranosyltransferase is an enzyme with systematic name UDP-alpha-D-galactofuranose:alpha-L-rhamnopyranosyl-(1->3)-N-acetyl-alpha-D-glucosaminyl-diphospho-trans,octacis-decaprenol 3-beta/4-beta-galactofuranosyltransferase. This enzyme catalyses the following chemical reaction
Lipid IVA 4-amino-4-deoxy-L-arabinosyltransferase is an enzyme with systematic name 4-amino-4-deoxy-alpha-L-arabinopyranosyl ditrans, octacis-undecaprenyl phosphate:lipid IVA 4-amino-4-deoxy-L-arabinopyranosyltransferase. This enzyme catalyses the following chemical reaction
Arabinofuranan 3-O-arabinosyltransferase is an enzyme with systematic name alpha-(1->5)-arabinofuranan:trans,octacis-decaprenylphospho-beta-D-arabinofuranose 3-O-alpha-D-arabinofuranosyltransferase. This enzyme catalyses the following chemical reaction:
Undecaprenyl-phosphate 4-deoxy-4-formamido-L-arabinose transferase is an enzyme with systematic name UDP-4-amino-4-deoxy-alpha-L-arabinose:ditrans,octacis-undecaprenyl phosphate 4-amino-4-deoxy-alpha-L-arabinosyltransferase. This enzyme catalyses the following chemical reaction














