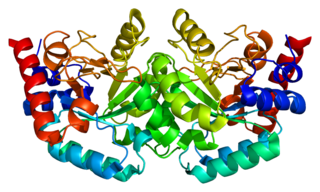
Uridine monophosphate synthase (UMPS) is the enzyme that catalyses the formation of uridine monophosphate (UMP), an energy-carrying molecule in many important biosynthetic pathways. In humans, the gene that codes for this enzyme is located on the long arm of chromosome 3 (3q13).
A salvage pathway is a pathway in which a biological product is produced from intermediates in the degradative pathway of its own or a similar substance. The term often refers to nucleotide salvage in particular, in which nucleotides are synthesized from intermediates in their degradative pathway.

Disodium guanylate, also known as sodium 5'-guanylate and disodium 5'-guanylate, is a natural sodium salt of the flavor enhancing nucleotide guanosine monophosphate (GMP). Disodium guanylate is a food additive with the E number E627. It is commonly used in conjunction with glutamic acid.

Hypoxanthine-guanine phosphoribosyltransferase (HGPRT) is an enzyme encoded in humans by the HPRT1 gene.
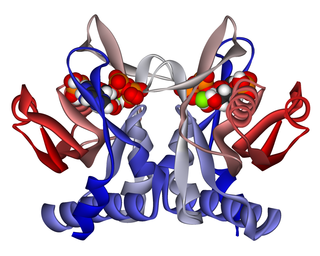
Adenine phosphoribosyltransferase (APRTase) is an enzyme encoded by the APRT gene, found in humans on chromosome 16. It is part of the Type I PRTase family and is involved in the nucleotide salvage pathway, which provides an alternative to nucleotide biosynthesis de novo in humans and most other animals. In parasitic protozoa such as giardia, APRTase provides the sole mechanism by which AMP can be produced. APRTase deficiency contributes to the formation of kidney stones (urolithiasis) and to potential kidney failure.

Adenine phosphoribosyltransferase deficiency is an autosomal recessive metabolic disorder associated with a mutation in the enzyme adenine phosphoribosyltransferase.
Pentosyltransferases are a type of glycosyltransferase that catalyze the transfer of a pentose.

2,8-Dihydroxyadenine is a derivative of adenine which accumulates in 2,8 dihydroxy-adenine urolithiasis. The poorly soluble purine 2,8-dihydroxyadenine is excreted in the urine because of a deficiency in the adenine salvage enzyme adenine phosphoribosyltransferase. The defect is inherited as an autosomal recessive trait; the homozygous state is associated with high urinary levels of 2,8-dihydroxyadenine and with crystalluria, calculus formation, and potential nephrotoxicity. The condition primarily presents as renal obstructive disease, but some patients have presented with advanced kidney failure. Allopurinol therapy appears to be effective. 2, 8-dihydroxyadenine formation can be easily controlled with allopurinol, which is administered in a dose of 300 mg/day in adults in the absence of kidney failure.

Orotidine 5'-monophosphate (OMP), also known as orotidylic acid, is a pyrimidine nucleotide which is the last intermediate in the biosynthesis of uridine monophosphate. OMP is formed from orotate and phosphoribosyl pyrophosphate by the enzyme orotate phosphoribosyltransferase

Orotate phosphoribosyltransferase (OPRTase) or orotic acid phosphoribosyltransferase is an enzyme involved in pyrimidine biosynthesis. It catalyzes the formation of orotidine 5'-monophosphate (OMP) from orotate and phosphoribosyl pyrophosphate. In yeast and bacteria, orotate phosphoribosyltransferase is an independent enzyme with a unique gene coding for the protein, whereas in mammals and other multicellular organisms, the catalytic function is carried out by a domain of the bifunctional enzyme UMP synthase (UMPS).
Purine metabolism refers to the metabolic pathways to synthesize and break down purines that are present in many organisms.

Amidophosphoribosyltransferase (ATase), also known as glutamine phosphoribosylpyrophosphate amidotransferase (GPAT), is an enzyme responsible for catalyzing the conversion of 5-phosphoribosyl-1-pyrophosphate (PRPP) into 5-phosphoribosyl-1-amine (PRA), using the amine group from a glutamine side-chain. This is the committing step in de novo purine synthesis. In humans it is encoded by the PPAT gene. ATase is a member of the purine/pyrimidine phosphoribosyltransferase family.
Uracil phosphoribosyltransferase is an enzyme which creates UMP from uracil and phosphoribosylpyrophosphate. This protein may use the morpheein model of allosteric regulation.

In enzymology, an anthranilate phosphoribosyltransferase is an enzyme that catalyzes the chemical reaction

In enzymology, an ATP phosphoribosyltransferase is an enzyme that catalyzes the chemical reaction

Nicotinamide phosphoribosyltransferase, formerly known as pre-B-cell colony-enhancing factor 1 (PBEF1) or visfatin for its extracellular form (eNAMPT), is an enzyme that in humans is encoded by the NAMPT gene. The intracellular form of this protein (iNAMPT) is the rate-limiting enzyme in the nicotinamide adenine dinucleotide (NAD+) salvage pathway that converts nicotinamide to nicotinamide mononucleotide (NMN) which is responsible for most of the NAD+ formation in mammals. iNAMPT can also catalyze the synthesis of NMN from phosphoribosyl pyrophosphate (PRPP) when ATP is present. eNAMPT has been reported to be a cytokine (PBEF) that activates TLR4, that promotes B cell maturation, and that inhibits neutrophil apoptosis.

In enzymology, a nicotinate-nucleotide-dimethylbenzimidazole phosphoribosyltransferase is an enzyme that catalyzes the chemical reaction

In enzymology, a nicotinate-nucleotide diphosphorylase (carboxylating) (EC 2.4.2.19) is an enzyme that catalyzes the chemical reaction

In enzymology, a nicotinate phosphoribosyltransferase (EC 6.3.4.21) is an enzyme that catalyzes the chemical reaction
Decaprenyl-phosphate phosphoribosyltransferase is an enzyme with systematic name trans,octacis-decaprenylphospho-beta-D-ribofuranose 5-phosphate:diphosphate phospho-alpha-D-ribosyltransferase. This enzyme catalyses the following chemical reaction
This page is based on this
Wikipedia article Text is available under the
CC BY-SA 4.0 license; additional terms may apply.
Images, videos and audio are available under their respective licenses.













