| |||
| |||
| Names | |||
|---|---|---|---|
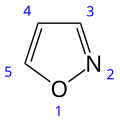
| Preferred IUPAC name 1,2-Oxazole [1] | |||
| Other names isoxazole | |||
| Identifiers | |||
3D model (JSmol) | |||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.005.472 | ||
PubChem CID | |||
| UNII | |||
CompTox Dashboard (EPA) | |||
| |||
| |||
| Properties | |||
| C3H3NO | |||
| Molar mass | 69.06202 g/mol | ||
| Density | 1.075 g/ml | ||
| Boiling point | 95 °C (203 °F; 368 K) | ||
| Acidity (pKa) | −3.0 (of conjugate acid) [2] | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
Isoxazole is an electron-rich azole with an oxygen atom next to the nitrogen. It is also the class of compounds containing this ring. Isoxazolyl is the univalent functional group derived from isoxazole.