This article may need to be rewritten to comply with Wikipedia's quality standards.(June 2025) |
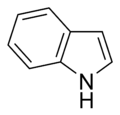
Simple aromatic rings, also known as simple arenes or simple aromatics, are aromatic organic compounds that consist only of a conjugated planar ring system.[ citation needed ] Many simple aromatic rings have trivial names. They are usually found as substructures of more complex molecules ("substituted aromatics"). Typical simple aromatic compounds are benzene, indole, and pyridine. [1] [ page needed ] [2] [ page needed ]
Contents
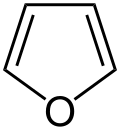
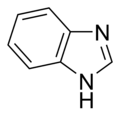
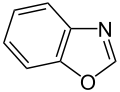
Simple aromatic rings can be heterocyclic if they contain non-carbon ring atoms, for example, oxygen, nitrogen, or sulfur. They can be monocyclic as in benzene, bicyclic as in naphthalene, or polycyclic as in anthracene. Simple monocyclic aromatic rings are usually five-membered rings like pyrrole or six-membered rings like pyridine. Fused bicyclic molecules consist of two rings that are connected by shared edges.[ clarification needed ]