
Nucleotides are organic molecules composed of a nitrogenous base, a pentose sugar and a phosphate. They serve as monomeric units of the nucleic acid polymers – deoxyribonucleic acid (DNA) and ribonucleic acid (RNA), both of which are essential biomolecules within all life-forms on Earth. Nucleotides are obtained in the diet and are also synthesized from common nutrients by the liver.
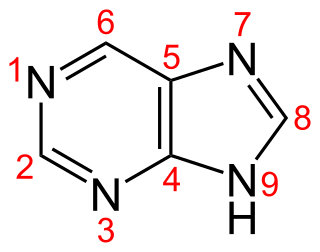
Purine is a heterocyclic aromatic organic compound that consists of two rings fused together. It is water-soluble. Purine also gives its name to the wider class of molecules, purines, which include substituted purines and their tautomers. They are the most widely occurring nitrogen-containing heterocycles in nature.
Pyrimidine is an aromatic, heterocyclic, organic compound similar to pyridine. One of the three diazines, it has nitrogen atoms at positions 1 and 3 in the ring. The other diazines are pyrazine and pyridazine.

Nucleobases are nitrogen-containing biological compounds that form nucleosides, which, in turn, are components of nucleotides, with all of these monomers constituting the basic building blocks of nucleic acids. The ability of nucleobases to form base pairs and to stack one upon another leads directly to long-chain helical structures such as ribonucleic acid (RNA) and deoxyribonucleic acid (DNA). Five nucleobases—adenine (A), cytosine (C), guanine (G), thymine (T), and uracil (U)—are called primary or canonical. They function as the fundamental units of the genetic code, with the bases A, G, C, and T being found in DNA while A, G, C, and U are found in RNA. Thymine and uracil are distinguished by merely the presence or absence of a methyl group on the fifth carbon (C5) of these heterocyclic six-membered rings. In addition, some viruses have aminoadenine (Z) instead of adenine. It differs in having an extra amine group, creating a more stable bond to thymine.
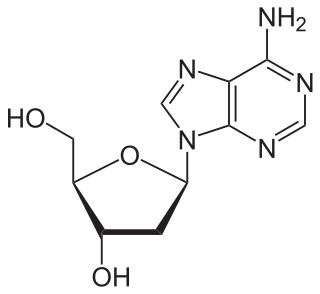
Nucleosides are glycosylamines that can be thought of as nucleotides without a phosphate group. A nucleoside consists simply of a nucleobase and a five-carbon sugar whereas a nucleotide is composed of a nucleobase, a five-carbon sugar, and one or more phosphate groups. In a nucleoside, the anomeric carbon is linked through a glycosidic bond to the N9 of a purine or the N1 of a pyrimidine. Nucleotides are the molecular building blocks of DNA and RNA.

In biochemistry, a ribonucleotide is a nucleotide containing ribose as its pentose component. It is considered a molecular precursor of nucleic acids. Nucleotides are the basic building blocks of DNA and RNA. Ribonucleotides themselves are basic monomeric building blocks for RNA. Deoxyribonucleotides, formed by reducing ribonucleotides with the enzyme ribonucleotide reductase (RNR), are essential building blocks for DNA. There are several differences between DNA deoxyribonucleotides and RNA ribonucleotides. Successive nucleotides are linked together via phosphodiester bonds.
A salvage pathway is a pathway in which a biological product is produced from intermediates in the degradative pathway of its own or a similar substance. The term often refers to nucleotide salvage in particular, in which nucleotides are synthesized from intermediates in their degradative pathway.

An imidazopyridine is a nitrogen containing heterocycle that is also a class of drugs that contain this same chemical substructure. In general, they are GABAA receptor agonists, however recently proton pump inhibitors, aromatase inhibitors, NSAIDs and other classes of drugs in this class have been developed as well. Despite usually being similar to them in effect, they are not chemically related to benzodiazepines. As such, GABAA-agonizing imidazopyridines, pyrazolopyrimidines, and cyclopyrrones are sometimes grouped together and referred to as "nonbenzodiazepines." Imidazopyridines include:
A nucleoside triphosphate is a nucleoside containing a nitrogenous base bound to a 5-carbon sugar, with three phosphate groups bound to the sugar. They are the molecular precursors of both DNA and RNA, which are chains of nucleotides made through the processes of DNA replication and transcription. Nucleoside triphosphates also serve as a source of energy for cellular reactions and are involved in signalling pathways.

Vidarabine or 9-β-D-arabinofuranosyladenine (ara-A) is an antiviral drug which is active against herpes simplex and varicella zoster viruses.

Triazines are a class of nitrogen-containing heterocycles. The parent molecules' molecular formula is C3H3N3. They exist in three isomeric forms, 1,3,5-triazines being common.

Cyanuric chloride is an organic compound with the formula (NCCl)3. This white solid is the chlorinated derivative of 1,3,5-triazine. It is the trimer of cyanogen chloride. Cyanuric chloride is the main precursor to the popular but controversial herbicide atrazine.
1,3,5-Triazine, also called s-triazine, is an organic chemical compound with the formula (HCN)3. It is a six-membered heterocyclic aromatic ring, one of several isomeric triazines. S-triazine—the "symmetric" isomer—and its derivatives are useful in a variety of applications.

2,4,6-Tris(trinitromethyl)-1,3,5-triazine is a chemical compound that is a derivative of triazine first prepared in 1995. It is synthesized by destructive nitration of 2,4,6-tricarboxyl-1,3,5-triazine. It is noteworthy for having more nitro groups than it does carbon atoms, thus potentially being useful as an oxygen source, or added to oxygen-poor explosives to increase their power.
The Ramberg–Bäcklund reaction is an organic reaction converting an α-halo sulfone into an alkene in presence of a base with extrusion of sulfur dioxide. The reaction is named after the two Swedish chemists Ludwig Ramberg and Birger Bäcklund. The carbanion formed by deprotonation gives an unstable episulfone that decomposes with elimination of sulfur dioxide. This elimination step is considered to be a concerted cheletropic extrusion.

Purine nucleoside phosphorylase, PNP, PNPase or inosine phosphorylase is an enzyme that in humans is encoded by the NP gene. It catalyzes the chemical reaction
Non-nucleoside reverse-transcriptase inhibitors (NNRTIs) are antiretroviral drugs used in the treatment of human immunodeficiency virus (HIV). NNRTIs inhibit reverse transcriptase (RT), an enzyme that controls the replication of the genetic material of HIV. RT is one of the most popular targets in the field of antiretroviral drug development.
Synthesis of nucleosides involves the coupling of a nucleophilic, heterocyclic base with an electrophilic sugar. The silyl-Hilbert-Johnson reaction, which employs silylated heterocyclic bases and electrophilic sugar derivatives in the presence of a Lewis acid, is the most common method for forming nucleosides in this manner.

In chemistry, hexahydro-1,3,5-triazine is a class of heterocyclic compounds with the formula (CH2NR)3. They are reduced derivatives of 1,3,5-triazine, which have the formula (CHN)3, a family of aromatic heterocycles. They are often called triazacyclohexanes or TACH's but this acronym is also applied to cis,cis-1,3,5-triaminocyclohexane.

5-Aza-7-deazaguanine or 2-aminoimidazo[1,2-a][1,3,5]triazin-4(1H)-one is a 5-Aza-7-deazapurine base that is an isomer of guanine. It is used as a nucleobase of hachimoji DNA, in which it pairs with 6-Amino-5-nitropyridin-2-one.













