
In biochemistry, a kinase is an enzyme that catalyzes the transfer of phosphate groups from high-energy, phosphate-donating molecules to specific substrates. This process is known as phosphorylation, where the high-energy ATP molecule donates a phosphate group to the substrate molecule. This transesterification produces a phosphorylated substrate and ADP. Conversely, it is referred to as dephosphorylation when the phosphorylated substrate donates a phosphate group and ADP gains a phosphate group. These two processes, phosphorylation and dephosphorylation, occur four times during glycolysis.

Thymidine, also known as deoxythymidine, deoxyribosylthymine, or thymine deoxyriboside, is a pyrimidine deoxynucleoside. Deoxythymidine is the DNA nucleoside T, which pairs with deoxyadenosine (A) in double-stranded DNA. In cell biology it is used to synchronize the cells in G1/early S phase. The prefix deoxy- is often left out since there are no precursors of thymine nucleotides involved in RNA synthesis.

Guanosine-5'-triphosphate (GTP) is a purine nucleoside triphosphate. It is one of the building blocks needed for the synthesis of RNA during the transcription process. Its structure is similar to that of the guanosine nucleoside, the only difference being that nucleotides like GTP have phosphates on their ribose sugar. GTP has the guanine nucleobase attached to the 1' carbon of the ribose and it has the triphosphate moiety attached to ribose's 5' carbon.
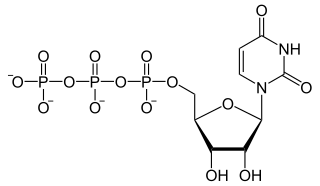
Uridine-5′-triphosphate (UTP) is a pyrimidine nucleoside triphosphate, consisting of the organic base uracil linked to the 1′ carbon of the ribose sugar, and esterified with tri-phosphoric acid at the 5′ position. Its main role is as substrate for the synthesis of RNA during transcription. UTP is the precursor for the production of CTP via CTP synthetase. UTP can be biosynthesized from UDP by Nucleoside Diphosphate Kinase after using the phosphate group from ATP. UDP + ATP ⇌ UTP + ADP; both UTP and ATP are energetically equal.
A salvage pathway is a pathway in which a biological product is produced from intermediates in the degradative pathway of its own or a similar substance. The term often refers to nucleotide salvage in particular, in which nucleotides are synthesized from intermediates in their degradative pathway.
A nucleoside triphosphate is a nucleoside containing a nitrogenous base bound to a 5-carbon sugar, with three phosphate groups bound to the sugar. They are the molecular precursors of both DNA and RNA, which are chains of nucleotides made through the processes of DNA replication and transcription. Nucleoside triphosphates also serve as a source of energy for cellular reactions and are involved in signalling pathways.

Valaciclovir, also spelled valacyclovir, is an antiviral medication used to treat outbreaks of herpes simplex or herpes zoster (shingles). It is also used to prevent cytomegalovirus following a kidney transplant in high risk cases. It is taken by mouth.

Thymidine kinase is an enzyme, a phosphotransferase : 2'-deoxythymidine kinase, ATP-thymidine 5'-phosphotransferase, EC 2.7.1.21. It can be found in most living cells. It is present in two forms in mammalian cells, TK1 and TK2. Certain viruses also have genetic information for expression of viral thymidine kinases. Thymidine kinase catalyzes the reaction:

Thymidine monophosphate (TMP), also known as thymidylic acid, deoxythymidine monophosphate (dTMP), or deoxythymidylic acid, is a nucleotide that is used as a monomer in DNA. It is an ester of phosphoric acid with the nucleoside thymidine. dTMP consists of a phosphate group, the pentose sugar deoxyribose, and the nucleobase thymine. Unlike the other deoxyribonucleotides, thymidine monophosphate often does not contain the "deoxy" prefix in its name; nevertheless, its symbol often includes a "d" ("dTMP"). Dorland’s Illustrated Medical Dictionary provides an explanation of the nomenclature variation at its entry for thymidine.

Thymidine diphosphate (TDP) or deoxythymidine diphosphate (dTDP) is a nucleotide diphosphate. It is an ester of pyrophosphoric acid with the nucleoside thymidine. dTDP consists of the pyrophosphate group, the pentose sugar ribose, and the nucleobase thymine. Unlike the other deoxyribonucleotides, thymidine diphosphate does not always contain the "deoxy" prefix in its name.

Nucleoside-diphosphate kinases are enzymes that catalyze the exchange of terminal phosphate between different nucleoside diphosphates (NDP) and triphosphates (NTP) in a reversible manner to produce nucleotide triphosphates. Many NDP serve as acceptor while NTP are donors of phosphate group. The general reaction via ping-pong mechanism is as follows: XDP + YTP ←→ XTP + YDP. NDPK activities maintain an equilibrium between the concentrations of different nucleoside triphosphates such as, for example, when guanosine triphosphate (GTP) produced in the citric acid (Krebs) cycle is converted to adenosine triphosphate (ATP). Other activities include cell proliferation, differentiation and development, signal transduction, G protein-coupled receptor, endocytosis, and gene expression.

The chemical compound 5-methyluridine, also called ribothymidine, is a pyrimidine nucleoside. It is the ribonucleoside counterpart to the deoxyribonucleoside thymidine, which lacks a hydroxyl group at the 2' position. 5-Methyluridine contains a thymine base joined to a ribose pentose sugar. It is a white solid.

Brivudine is an antiviral drug used in the treatment of herpes zoster ("shingles"). Like other antivirals, it acts by inhibiting replication of the target virus.

Penciclovir is a guanosine analogue antiviral drug used for the treatment of various herpesvirus infections. It is a nucleoside analogue which exhibits low toxicity and good selectivity. Because penciclovir is absorbed poorly when given orally it is more often used as a topical treatment. It is the active ingredient in the cold sore medications Denavir, Vectavir and Fenivir. Famciclovir is a prodrug of penciclovir with improved oral bioavailability.

Nucleic acid metabolism is a collective term that refers to the variety of chemical reactions by which nucleic acids are either synthesized or degraded. Nucleic acids are polymers made up of a variety of monomers called nucleotides. Nucleotide synthesis is an anabolic mechanism generally involving the chemical reaction of phosphate, pentose sugar, and a nitrogenous base. Degradation of nucleic acids is a catabolic reaction and the resulting parts of the nucleotides or nucleobases can be salvaged to recreate new nucleotides. Both synthesis and degradation reactions require multiple enzymes to facilitate the event. Defects or deficiencies in these enzymes can lead to a variety of diseases.

Deoxycytidine kinase (dCK) is an enzyme which is encoded by the DCK gene in humans. dCK predominantly phosphorylates deoxycytidine (dC) and converts dC into deoxycytidine monophosphate. dCK catalyzes one of the initial steps in the nucleoside salvage pathway and has the potential to phosphorylate other preformed nucleosides, specifically deoxyadenosine (dA) and deoxyguanosine (dG), and convert them into their monophosphate forms. There has been recent biomedical research interest in investigating dCK's potential as a therapeutic target for different types of cancer.
Discovery and development of nucleoside and nucleotide reverse-transcriptase inhibitors began in the 1980s when the AIDS epidemic hit Western societies. NRTIs inhibit the reverse transcriptase (RT), an enzyme that controls the replication of the genetic material of the human immunodeficiency virus (HIV). The first NRTI was zidovudine, approved by the U.S. Food and Drug Administration (FDA) in 1987, which was the first step towards treatment of HIV. Six NRTI agents and one NtRTI have followed. The NRTIs and the NtRTI are analogues of endogenous 2´-deoxy-nucleoside and nucleotide. Drug-resistant viruses are an inevitable consequence of prolonged exposure of HIV-1 to anti-HIV drugs.

Deoxythymidine triphosphate (dTTP) is one of the four nucleoside triphosphates that are used in the in vivo synthesis of DNA. Unlike the other deoxyribonucleoside triphosphates, thymidine triphosphate does not always contain the "deoxy" prefix in its name. The corresponding ribonucleoside triphosphate is called uridine triphosphate.

Trifluridine/tipiracil, sold under the brand name Lonsurf, is a fixed-dose combination medication that is used as a third- or fourth-line treatment of metastatic colorectal cancer or gastric cancer, after chemotherapy and targeted therapeutics have failed. It is a combination of two active pharmaceutical ingredients: trifluridine, a nucleoside analog, and tipiracil, a thymidine phosphorylase inhibitor. Tipiracil prevents rapid metabolism of trifluridine, increasing the bioavailability of trifluridine.

Doxifluridine is a second generation nucleoside analog prodrug developed by Roche and used as a cytostatic agent in chemotherapy in several Asian countries including China and South Korea. Doxifluridine is not FDA-approved for use in the USA. It is currently being evaluated in several clinical trials as a stand-alone or combination therapy treatment.


















