Contents
 | |
 | |
| Names | |
|---|---|
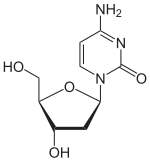
| IUPAC name 2′-deoxycytidine | |
| Systematic IUPAC name 4-Amino-1-[(2R,4S,5R)-4-hydroxy-5-(hydroxymethyl)oxolan-2-yl]pyrimidin-2(1H)-one | |
| Other names doxecitine | |
| Identifiers | |
3D model (JSmol) | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.012.231 |
| MeSH | Deoxycytidine |
PubChem CID | |
| UNII | |
CompTox Dashboard (EPA) | |
| |
| |
| Properties | |
| C9H13N3O4 | |
| Molar mass | 227.217 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Deoxycytidine is a deoxyribonucleoside, a component of deoxyribonucleic acid. It is similar to the ribonucleoside cytidine, but with one hydroxyl group removed from the C2' position. Deoxycytidine can be phosphorylated at C5' of the deoxyribose by deoxycytidine kinase, converting it to deoxycytidine monophosphate (dCMP), a DNA precursor. [1] dCMP can be converted to dUMP and dTMP.[ citation needed ]
Doxecitine is the international nonproprietary name. [2]