
A heterocyclic compound or ring structure is a cyclic compound that has atoms of at least two different elements as members of its ring(s). Heterocyclic chemistry is the branch of organic chemistry dealing with the synthesis, properties, and applications of these heterocycles.
The quinones are a class of organic compounds that are formally "derived from aromatic compounds [such as benzene or naphthalene] by conversion of an even number of –CH= groups into –C(=O)– groups with any necessary rearrangement of double bonds, resulting in "a fully conjugated cyclic dione structure". The archetypical member of the class is 1,4-benzoquinone or cyclohexadienedione, often called simply "quinone". Other important examples are 1,2-benzoquinone (ortho-quinone), 1,4-naphthoquinone and 9,10-anthraquinone.

Indazole, also called isoindazole, is a heterocyclic aromatic organic compound. This bicyclic compound consists of the fusion of benzene and pyrazole.
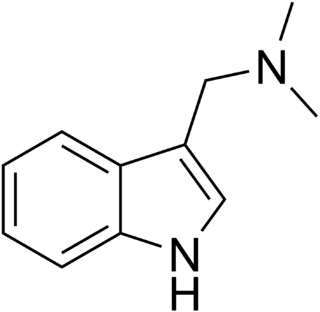
Gramine is a naturally occurring indole alkaloid present in several plant species. Gramine may play a defensive role in these plants, since it is toxic to many organisms.
Hydrazides in organic chemistry are a class of organic compounds with the formula RNHNH2 where R is acyl (R'CO-), sulfonyl (R'SO2-), or phosphoryl (R'2P(O)-). Unlike hydrazine and alkylhydrazines, hydrazides are nonbasic owing to the inductive influence of the acyl, sulfonyl, or phosphoryl substituent.

Pyridazine is a heterocyclic organic compound with the molecular formula (CH)4N2. It contains a six-membered ring with two adjacent nitrogen atoms, and is aromatic. It is a colorless liquid with a boiling point of 208 °C. It is isomeric with two other (CH)4N2 rings, pyrimidine and pyrazine.

Indoline is an aromatic heterocyclic organic compound with the chemical formula C8H9N. It has a bicyclic structure, consisting of a six-membered benzene ring fused to a five-membered nitrogen-containing ring. The compound is based on the indole structure, but the 2-3 bond is saturated. By oxidation/dehydrogenation it can be converted to indoles.

Carbazole is an aromatic heterocyclic organic compound. It has a tricyclic structure, consisting of two six-membered benzene rings fused on either side of a five-membered nitrogen-containing ring. The compound's structure is based on the indole structure, but in which a second benzene ring is fused onto the five-membered ring at the 2–3 position of indole.

Plakohypaphorines are halogenated indolic non-proteinogenic amino acids named for their similarity to hypaphorine (N,N,N-trimethyltryptophan), First reported in the Caribbean sponge Plakortis simplex in 2003, plakohypaphorines A-C were the first iodine-containing indoles to be discovered in nature. Plakohypaphorines D-F, also found in P. simplex, were reported in 2004 by a group including the researchers who discovered the original plakohypaphorines.

Pericine is one of a number of indole alkaloids found in the tree Picralima nitida, commonly known as akuamma. As with some other alkaloids from this plant such as akuammine, pericine has been shown to bind to mu opioid receptors in vitro, and has an IC50 of 0.6 μmol, within the range of a weak analgesic. It may also have convulsant effects.

Asima Chatterjee was an Indian organic chemist noted for her work in the fields of organic chemistry and phytomedicine. Her most notable work includes research on vinca alkaloids, the development of anti-epileptic drugs, and development of anti-malarial drugs. She also authored a considerable volume of work on medicinal plants of the Indian subcontinent. She was the first woman to receive a Doctorate of Science from an Indian university.

Indolizine(Chemical formula C8H7N) is a heterocyclic aromatic organic compound that is an isomer of indole. The saturated analog indolizidine forms the structural core of a variety of alkaloids such as swainsonine.

Indole is an aromatic heterocyclic organic compound with formula C8H7N. It has a bicyclic structure, consisting of a six-membered benzene ring fused to a five-membered pyrrole ring. Indole is widely distributed in the natural environment and can be produced by a variety of bacteria. As an intercellular signal molecule, indole regulates various aspects of bacterial physiology, including spore formation, plasmid stability, resistance to drugs, biofilm formation, and virulence. The amino acid tryptophan is an indole derivative and the precursor of the neurotransmitter serotonin.
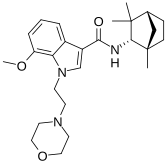
MN-25 (UR-12) is a drug invented by Bristol-Myers Squibb, that acts as a reasonably selective agonist of peripheral cannabinoid receptors. It has moderate affinity for CB2 receptors with a Ki of 11 nM, but 22x lower affinity for the psychoactive CB1 receptors with a Ki of 245 nM. The indole 2-methyl derivative has the ratio of affinities reversed however, with a Ki of 8 nM at CB1 and 29 nM at CB2, which contrasts with the usual trend of 2-methyl derivatives having increased selectivity for CB2 (cf. JWH-018 vs JWH-007, JWH-081 vs JWH-098).

Akuammicine is a monoterpene indole alkaloid of the Vinca sub-group. It is found in the Apocynaceae family of plants including Picralima nitida, Vinca minor and the Aspidosperma.
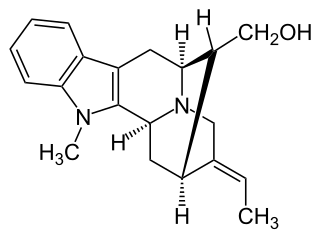
Affinisine is a monoterpenoid indole alkaloid which can be isolated from plants of the genus Tabernaemontana. Structurally it can be considered a member of the sarpagine alkaloid family and may be synthesized from tryptophan via a Pictet-Spengler reaction.
A ring forming reaction or ring-closing reaction in organic chemistry is a general term for a variety of reactions that introduce one or more rings into a molecule. A heterocycle forming reaction is such a reaction that introduces a new heterocycle. Important classes of ring forming reactions include annulations and cycloadditions.

Apparicine is a monoterpenoid indole alkaloid. It is named after Apparicio Duarte, a Brazilian botanist who studied the Aspidosperma species from which apparicine was first isolated. It was the first member of the vallesamine group of alkaloids to be isolated and have its structure established, which was first published in 1965. It has also been known by the synonyms gomezine, pericalline, and tabernoschizine.

LY-2452473 (TT-701) is a drug which acts as a selective androgen receptor modulator (SARM). It has been investigated for the treatment of erectile dysfunction and symptoms associated with benign prostate hyperplasia.
The Cadogan-Sundberg indole synthesis, or simply Cadogan indole synthesis, is a name reaction in organic chemistry that allows for the generation of indoles from o-nitrostyrenes with the use of trialkyl phosphites, such as triethyl phosphite.
















